当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Introduction of substituents for tuning the redox properties of benzoate-bridged paddlewheel diruthenium(II,II) complexes: what does the OH group bring?
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-12-12 , DOI: 10.1039/d4dt03020f Wataru Kosaka, Yudai Watanabe, Taku Kitayama, Chisa Itoh, Hitoshi Miyasaka
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-12-12 , DOI: 10.1039/d4dt03020f Wataru Kosaka, Yudai Watanabe, Taku Kitayama, Chisa Itoh, Hitoshi Miyasaka
|
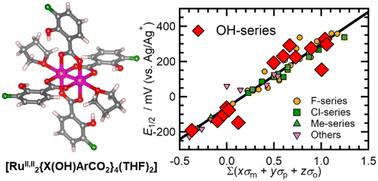
Benzoate-bridged paddlewheel diruthenium(II,II) complexes ([RuII,II2(RnArCO2)4(Lax)2] (Lax = axial ligand); [RuII,II2]) exhibit reversible redox activity involving the oxidized species [RuII,III2]+. The redox activity can be finely tuned over a broad potential range by altering the substituent R on the benzoate-bridging ligand RnArCO2−. The electronic contributions of the substituents R depend on their type and position, as was empirically demonstrated by Hammett for substituents at the meta- and para-positions. However, the substituent effect at the ortho-position is not solely determined by the electronic contribution of R but also by steric hindrance between the o-substituents and adjacent carboxylate groups. Nevertheless, an OH group at the o-position did not provide any steric hindrance, leading to a strong electron-withdrawing effect owing to intramolecular hydrogen bonding between the o-OH group and the adjacent carboxylate group, despite the electron-donating ability of the m- and p-OH groups. The OH group at the o-position induced a significant shift in the redox potential and HOMO energy levels of the [RuII,II2] complexes, thereby stabilizing the [RuII,II2] state. The redox potential and HOMO can be adjusted by introducing additional substituents, such as F, Cl, Me, OMe, and CF3 groups, to cover a wide range, in accordance with an extended Hammett law that considers the contribution of the o-position.
中文翻译:

介绍用于调节苯甲酸盐桥式桨轮二钌 (II,II) 配合物氧化还原性能的取代基:OH 基团带来什么?
苯甲酸盐桥式桨轮二钌 (II,II) 配合物 ([RuII,II2(RnArCO2)4(Lax)2] (Lax = 轴向配体);[RuII,II2])表现出涉及氧化物质 [RuII,III2]+ 的可逆氧化还原活性。通过改变苯甲酸盐桥接配体 RnArCO2− 上的取代基 R,可以在很宽的电位范围内微调氧化还原活性。取代基 R 的电子贡献取决于它们的类型和位置,正如 Hammett 对位位和对位的取代基的经验证明的那样。然而,邻位的取代基效应不仅取决于 R 的电子贡献,还取决于 o 取代基和相邻羧酸盐基团之间的空间位阻。然而,尽管 m- 和 p-OH 基团具有电子供体能力,但 o 位的 OH 基团没有提供任何空间位阻,由于 o-OH 基团和相邻羧酸盐基团之间的分子内氢键,导致强大的吸电子效应。o 位的 OH 基团诱导 [RuII,II2] 配合物的氧化还原电位和 HOMO 能级发生显着变化,从而稳定 [RuII,II2] 状态。 氧化还原电位和 HOMO 可以通过引入额外的取代基(如 F、Cl、Me、OMe 和 CF3 基团)来调整,以覆盖较宽的范围,符合考虑 o 位置贡献的扩展哈米特定律。
更新日期:2024-12-16
中文翻译:

介绍用于调节苯甲酸盐桥式桨轮二钌 (II,II) 配合物氧化还原性能的取代基:OH 基团带来什么?
苯甲酸盐桥式桨轮二钌 (II,II) 配合物 ([RuII,II2(RnArCO2)4(Lax)2] (Lax = 轴向配体);[RuII,II2])表现出涉及氧化物质 [RuII,III2]+ 的可逆氧化还原活性。通过改变苯甲酸盐桥接配体 RnArCO2− 上的取代基 R,可以在很宽的电位范围内微调氧化还原活性。取代基 R 的电子贡献取决于它们的类型和位置,正如 Hammett 对位位和对位的取代基的经验证明的那样。然而,邻位的取代基效应不仅取决于 R 的电子贡献,还取决于 o 取代基和相邻羧酸盐基团之间的空间位阻。然而,尽管 m- 和 p-OH 基团具有电子供体能力,但 o 位的 OH 基团没有提供任何空间位阻,由于 o-OH 基团和相邻羧酸盐基团之间的分子内氢键,导致强大的吸电子效应。o 位的 OH 基团诱导 [RuII,II2] 配合物的氧化还原电位和 HOMO 能级发生显着变化,从而稳定 [RuII,II2] 状态。 氧化还原电位和 HOMO 可以通过引入额外的取代基(如 F、Cl、Me、OMe 和 CF3 基团)来调整,以覆盖较宽的范围,符合考虑 o 位置贡献的扩展哈米特定律。






























 京公网安备 11010802027423号
京公网安备 11010802027423号