当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile access to chiral anti-1,2-diol derivatives via Ir-catalyzed asymmetric hydrogenation of α-alkoxy-β-ketoesters
Chemical Communications ( IF 4.3 ) Pub Date : 2024-12-12 , DOI: 10.1039/d4cc05644b Yuan-Zheng Wang, Bin Lu, Gen-Qiang Chen, Xumu Zhang
Chemical Communications ( IF 4.3 ) Pub Date : 2024-12-12 , DOI: 10.1039/d4cc05644b Yuan-Zheng Wang, Bin Lu, Gen-Qiang Chen, Xumu Zhang
|
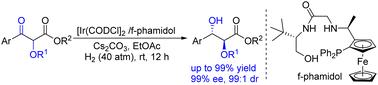
The iridium-catalyzed asymmetric hydrogenation of α-alkoxy-β-ketoesters via dynamic kinetic resolution has been achieved with high efficiency and enantioselectivity. This strategy allows for the synthesis of differentiated anti-1,2-diol derivatives in high yields, exhibiting excellent enantio- and diastereoselectivity (up to 99% yield, 99% ee, and 99 : 1 dr). Additionally, high turnover number (TON) experiments (up to 1000 TON) and gram-scale synthesis of a key fragment of the potential drug Tesaglitazar were successfully performed, highlighting the protocol's potential for broader applications.
中文翻译:

通过 Ir 催化的 α-烷氧基-β-酮酯的不对称氢化轻松获得手性抗 1,2-二醇衍生物
通过动态动力学分辨率实现铱催化的 α-烷氧基-β-酮酯的不对称氢化,具有很高的效率和对映选择性。该策略允许以高产率合成差异化的抗 1,2-二醇衍生物,表现出优异的对映和非对映选择性(高达 99% 的产率、99% 的 ee 和 99 : 1 dr)。此外,成功进行了高周转数 (TON) 实验(高达 1000 TON)和潜在药物 Tesaglitazar 关键片段的克级合成,突出了该方案具有更广泛应用的潜力。
更新日期:2024-12-12
中文翻译:

通过 Ir 催化的 α-烷氧基-β-酮酯的不对称氢化轻松获得手性抗 1,2-二醇衍生物
通过动态动力学分辨率实现铱催化的 α-烷氧基-β-酮酯的不对称氢化,具有很高的效率和对映选择性。该策略允许以高产率合成差异化的抗 1,2-二醇衍生物,表现出优异的对映和非对映选择性(高达 99% 的产率、99% 的 ee 和 99 : 1 dr)。此外,成功进行了高周转数 (TON) 实验(高达 1000 TON)和潜在药物 Tesaglitazar 关键片段的克级合成,突出了该方案具有更广泛应用的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号