当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Acyl-N-alkyl/aryl Sulfonamide Chemistry Assisted by Proximity for Modification and Covalent Inhibition of Endogenous Proteins in Living Systems
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-12-11 , DOI: 10.1021/acs.accounts.4c00628 Tomonori Tamura, Itaru Hamachi
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-12-11 , DOI: 10.1021/acs.accounts.4c00628 Tomonori Tamura, Itaru Hamachi
|
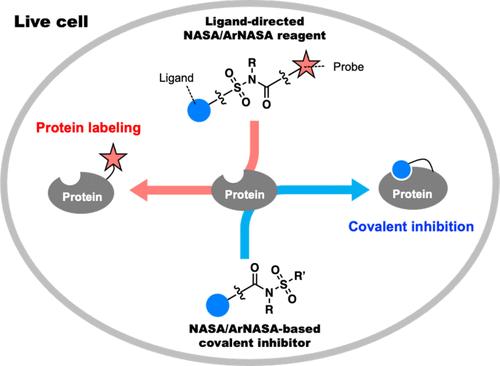
Selective chemical modification of endogenous proteins in living systems with synthetic small molecular probes is a central challenge in chemical biology. Such modification has a variety of applications important for biological and pharmaceutical research, including protein visualization, protein functionalization, proteome-wide profiling of enzyme activity, and irreversible inhibition of protein activity. Traditional chemistry for selective protein modification in cells largely relies on the high nucleophilicity of cysteine residues to ensure target-selectivity and site-specificity of modification. More recently, lysine residues, which are more abundant on protein surfaces, have attracted attention for the covalent modification of proteins. However, it has been difficult to efficiently modify the ε-amino groups of lysine side-chains, which are mostly (∼99.9%) protonated and thus exhibit low nucleophilicity at physiological pH. Our group revealed that N-acyl-N-alkyl sulfonamide (NASA) moieties can rapidly and efficiently acylate noncatalytic (i.e., less reactive) lysine residues in proteins by leveraging a reaction acceleration effect via proximity. The excellent reaction kinetics and selectivity for lysine of the NASA chemistry enable covalent modification of natural intracellular and cell-surface proteins, which is intractable using conventional chemistries. Moreover, recently developed N-acyl-N-aryl sulfonamide (ArNASA) scaffolds overcome some problems faced by the first-generation NASA compounds. In this Account, we summarize our recent works in the development of NASA/ArNASA chemistry and several applications reported by ourselves and other groups. First, we characterize the basic properties of NASA/ArNASA chemistry, including the labeling kinetics, amino acid preference, and biocompatibility, and compare this approach with other ligand-directed chemistries. This section also describes the principles of nucleophilic organocatalyst-mediated protein acylation, another important protein labeling strategy using the NASA reactive group, and its application to neurotransmitter receptor labeling in brain slices. Second, we highlight various recent examples of protein functionalization using NASA/ArNASA chemistry, such as visualization of membrane proteins including therapeutically important G-protein coupled receptors, gel-based ligand screening assays, photochemical control of protein activity, and targeted protein degradation. Third, we survey covalent inhibition of proteins by NASA/ArNASA-based lysine-targeting. The unprecedented reactivity of NASA/ArNASA toward lysine allows highly potent, irreversible inhibition of several drug targets for the treatment of cancer, including HSP90, HDM2–p53 protein–protein interaction, and a Bruton’s tyrosine kinase mutant that has developed resistance to cysteine-targeted covalent-binding drugs. Finally, current limitations of, and future perspectives on, this research field are discussed. The new chemical labeling techniques offered by NASA/ArNASA chemistry and its derivatives create a valuable molecular toolbox for studying numerous biomolecules in living cells and even in vivo.
中文翻译:

N-酰基-N-烷基/芳基磺酰胺化学在邻近条件下对生命系统中内源性蛋白质的修饰和共价抑制
使用合成小分子探针对生命系统中的内源性蛋白质进行选择性化学修饰是化学生物学的一个核心挑战。这种修饰具有多种重要的生物学和药物研究应用,包括蛋白质可视化、蛋白质功能化、酶活性的蛋白质组范围分析以及蛋白质活性的不可逆抑制。用于细胞中选择性蛋白质修饰的传统化学在很大程度上依赖于半胱氨酸残基的高亲核性,以确保修饰的靶标选择性和位点特异性。最近,蛋白质表面更丰富的赖氨酸残基因蛋白质的共价修饰而引起人们的关注。然而,很难有效地修饰赖氨酸侧链的 ε-氨基,这些侧链大多 (∼99.9%) 质子化,因此在生理 pH 值下表现出低亲核性。我们小组发现,N-酰基-N-烷基磺酰胺 (NASA) 部分可以通过邻近利用反应加速效应,快速有效地酰化蛋白质中的非催化(即反应性较低)赖氨酸残基。NASA 化学试剂对赖氨酸的出色反应动力学和选择性使天然细胞内和细胞表面蛋白的共价修饰成为可能,而使用传统化学试剂很难做到这一点。此外,最近开发的 N-酰基-N-芳基磺酰胺 (ArNASA) 支架克服了第一代 NASA 化合物面临的一些问题。在本账户中,我们总结了我们最近在 NASA/ArNASA 化学开发方面的工作以及我们自己和其他小组报告的几个应用。 首先,我们表征了 NASA/ArNASA 化学的基本性质,包括标记动力学、氨基酸偏好和生物相容性,并将这种方法与其他配体定向化学进行比较。本节还描述了亲核有机催化剂介导的蛋白质酰化的原理,另一种使用 NASA 反应基团的重要蛋白质标记策略,及其在脑切片中神经递质受体标记中的应用。其次,我们重点介绍了使用 NASA/ArNASA 化学进行蛋白质功能化的各种最新实例,例如膜蛋白的可视化,包括具有治疗意义的 G 蛋白偶联受体、基于凝胶的配体筛选测定、蛋白质活性的光化学控制和靶向蛋白质降解。第三,我们研究了基于 NASA/ArNASA 的赖氨酸靶向对蛋白质的共价抑制。NASA/ArNASA 对赖氨酸的空前反应性允许对治疗癌症的多种药物靶点进行高效、不可逆的抑制,包括 HSP90、HDM2-p53 蛋白-蛋白质相互作用,以及对半胱氨酸靶向共价结合药物产生耐药性的布鲁顿酪氨酸激酶突变体。最后,讨论了该研究领域的当前局限性和未来前景。NASA/ArNASA 化学及其衍生物提供的新型化学标记技术为研究活细胞甚至体内的众多生物分子创造了一个有价值的分子工具箱。
更新日期:2024-12-12
中文翻译:

N-酰基-N-烷基/芳基磺酰胺化学在邻近条件下对生命系统中内源性蛋白质的修饰和共价抑制
使用合成小分子探针对生命系统中的内源性蛋白质进行选择性化学修饰是化学生物学的一个核心挑战。这种修饰具有多种重要的生物学和药物研究应用,包括蛋白质可视化、蛋白质功能化、酶活性的蛋白质组范围分析以及蛋白质活性的不可逆抑制。用于细胞中选择性蛋白质修饰的传统化学在很大程度上依赖于半胱氨酸残基的高亲核性,以确保修饰的靶标选择性和位点特异性。最近,蛋白质表面更丰富的赖氨酸残基因蛋白质的共价修饰而引起人们的关注。然而,很难有效地修饰赖氨酸侧链的 ε-氨基,这些侧链大多 (∼99.9%) 质子化,因此在生理 pH 值下表现出低亲核性。我们小组发现,N-酰基-N-烷基磺酰胺 (NASA) 部分可以通过邻近利用反应加速效应,快速有效地酰化蛋白质中的非催化(即反应性较低)赖氨酸残基。NASA 化学试剂对赖氨酸的出色反应动力学和选择性使天然细胞内和细胞表面蛋白的共价修饰成为可能,而使用传统化学试剂很难做到这一点。此外,最近开发的 N-酰基-N-芳基磺酰胺 (ArNASA) 支架克服了第一代 NASA 化合物面临的一些问题。在本账户中,我们总结了我们最近在 NASA/ArNASA 化学开发方面的工作以及我们自己和其他小组报告的几个应用。 首先,我们表征了 NASA/ArNASA 化学的基本性质,包括标记动力学、氨基酸偏好和生物相容性,并将这种方法与其他配体定向化学进行比较。本节还描述了亲核有机催化剂介导的蛋白质酰化的原理,另一种使用 NASA 反应基团的重要蛋白质标记策略,及其在脑切片中神经递质受体标记中的应用。其次,我们重点介绍了使用 NASA/ArNASA 化学进行蛋白质功能化的各种最新实例,例如膜蛋白的可视化,包括具有治疗意义的 G 蛋白偶联受体、基于凝胶的配体筛选测定、蛋白质活性的光化学控制和靶向蛋白质降解。第三,我们研究了基于 NASA/ArNASA 的赖氨酸靶向对蛋白质的共价抑制。NASA/ArNASA 对赖氨酸的空前反应性允许对治疗癌症的多种药物靶点进行高效、不可逆的抑制,包括 HSP90、HDM2-p53 蛋白-蛋白质相互作用,以及对半胱氨酸靶向共价结合药物产生耐药性的布鲁顿酪氨酸激酶突变体。最后,讨论了该研究领域的当前局限性和未来前景。NASA/ArNASA 化学及其衍生物提供的新型化学标记技术为研究活细胞甚至体内的众多生物分子创造了一个有价值的分子工具箱。






























 京公网安备 11010802027423号
京公网安备 11010802027423号