当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and evaluation of novel benzofuran and pyrazole-based derivatives as dual AChE/BuChE inhibitors with antioxidant properties for Alzheimer's disease management
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-10 , DOI: 10.1016/j.ejmech.2024.117158 Mahmoud S. Elkotamy, Mohamed K. Elgohary, Mahmoud Abdelrahman Alkabbani, Mohamed M. Hefina, Haytham O. Tawfik, Mohamed Fares, Wagdy M. Eldehna, Hatem A. Abdel-Aziz
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-10 , DOI: 10.1016/j.ejmech.2024.117158 Mahmoud S. Elkotamy, Mohamed K. Elgohary, Mahmoud Abdelrahman Alkabbani, Mohamed M. Hefina, Haytham O. Tawfik, Mohamed Fares, Wagdy M. Eldehna, Hatem A. Abdel-Aziz
|
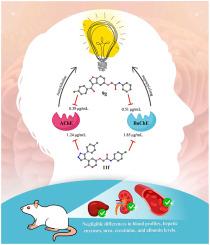
As a complicated neurodegenerative disorder with several clinical hallmarks, Alzheimer's disease (AD) requires multi-target treatment medicines to address multiple elements of disease progression. In this study, we reported two novel series of compounds: benzofuran-based donepezil analogs (9a-i ) and their pyrazole-based counterparts (11a-i ) as potential dual inhibitors of AChE and BuChE with additional antioxidant properties, aiming to address multiple pathological aspects of AD simultaneously. The design strategy employed bioisosteric replacement, substituting donepezil's indanone motif with a benzofuran ring in series (9a-i ) to maintain crucial hydrogen bonding interactions with the Phe295 residue in the enzyme's active site. Subsequently, the benzofuran ring underwent cleavage, yielding pyrazole-tethered hydroxyphenyl derivatives (11a-i ). The biological evaluation revealed that benzofuran-based derivative 9g exhibited exceptional efficacy against both AChE and BuChE, with IC50 values of 0.39 and 0.51 μg/ml, respectively, although it lacked antioxidant activity. Compound 11f demonstrated dual inhibition of AChE (IC50 = 1.24 μg/ml) and BuChE (IC50 = 1.85 μg/ml) while also displaying strong DPPH free radical scavenging activity (IC50 = 3.15 μg/ml). In vivo toxicity studies on compound 11f revealed a favorable safety profile, with no signs of toxicity or adverse events in acute oral toxicity tests in male Wistar rats. Chronic administration of 11f resulted in negligible differences in blood profiles, hepatic enzymes, urea, creatinine, and albumin levels compared to the control group. Histopathological examination of hepatic and kidney tissues from treated rats showed normal histology without damage. In silico molecular docking analysis was performed to rationalize the design approaches and support the experimental findings. This study provides valuable insights into the development of multi-target compounds for potential Alzheimer's disease treatment.
中文翻译:

设计、合成和评价新型苯并呋喃和吡唑类衍生物作为具有抗氧化特性的双重 AChE/BuChE 抑制剂,用于阿尔茨海默病管理
阿尔茨海默病 (AD) 是一种具有多种临床特征的复杂神经退行性疾病,需要多靶点治疗药物来解决疾病进展的多个因素。在这项研究中,我们报道了两个新的化合物系列:基于苯并呋喃的多奈哌齐类似物 (9a-i) 及其基于吡唑的对应物 (11a-i) 作为具有额外抗氧化特性的潜在 AChE 和 BuChE 双重抑制剂,旨在同时解决 AD 的多个病理方面。该设计策略采用生物等排置换,用串联的苯并呋喃环 (9a-i) 取代多奈哌齐的茚酮基序,以维持与酶活性位点中 Phe295 残基的关键氢键相互作用。随后,苯并呋喃环发生裂解,产生吡唑-栓系羟苯衍生物 (11a-i)。生物学评价显示,基于苯并呋喃的衍生物 9g 对 AChE 和 BuChE 均表现出非凡的疗效,IC50 值分别为 0.39 和 0.51 μg/ml,尽管它缺乏抗氧化活性。化合物 11f 对 AChE (IC50 = 1.24 μg/ml) 和 BuChE (IC50 = 1.85 μg/ml) 具有双重抑制作用,同时还表现出很强的 DPPH 自由基清除活性 (IC50 = 3.15 μg/ml)。化合物 11f 的体内毒性研究显示出良好的安全性,在雄性 Wistar 大鼠的急性口服毒性试验中没有毒性或不良事件的迹象。与对照组相比,长期服用 11f 导致血谱、肝酶、尿素、肌酐和白蛋白水平的差异可以忽略不计。治疗大鼠肝肾组织的组织病理学检查显示组织学正常,无损伤。 进行计算机分子对接分析以合理化设计方法并支持实验结果。本研究为开发用于潜在阿尔茨海默病治疗的多靶点化合物提供了有价值的见解。
更新日期:2024-12-10
中文翻译:

设计、合成和评价新型苯并呋喃和吡唑类衍生物作为具有抗氧化特性的双重 AChE/BuChE 抑制剂,用于阿尔茨海默病管理
阿尔茨海默病 (AD) 是一种具有多种临床特征的复杂神经退行性疾病,需要多靶点治疗药物来解决疾病进展的多个因素。在这项研究中,我们报道了两个新的化合物系列:基于苯并呋喃的多奈哌齐类似物 (9a-i) 及其基于吡唑的对应物 (11a-i) 作为具有额外抗氧化特性的潜在 AChE 和 BuChE 双重抑制剂,旨在同时解决 AD 的多个病理方面。该设计策略采用生物等排置换,用串联的苯并呋喃环 (9a-i) 取代多奈哌齐的茚酮基序,以维持与酶活性位点中 Phe295 残基的关键氢键相互作用。随后,苯并呋喃环发生裂解,产生吡唑-栓系羟苯衍生物 (11a-i)。生物学评价显示,基于苯并呋喃的衍生物 9g 对 AChE 和 BuChE 均表现出非凡的疗效,IC50 值分别为 0.39 和 0.51 μg/ml,尽管它缺乏抗氧化活性。化合物 11f 对 AChE (IC50 = 1.24 μg/ml) 和 BuChE (IC50 = 1.85 μg/ml) 具有双重抑制作用,同时还表现出很强的 DPPH 自由基清除活性 (IC50 = 3.15 μg/ml)。化合物 11f 的体内毒性研究显示出良好的安全性,在雄性 Wistar 大鼠的急性口服毒性试验中没有毒性或不良事件的迹象。与对照组相比,长期服用 11f 导致血谱、肝酶、尿素、肌酐和白蛋白水平的差异可以忽略不计。治疗大鼠肝肾组织的组织病理学检查显示组织学正常,无损伤。 进行计算机分子对接分析以合理化设计方法并支持实验结果。本研究为开发用于潜在阿尔茨海默病治疗的多靶点化合物提供了有价值的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号