当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological evaluation of steroidal indole derivatives as membrane-targeting antibacterial candidates
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-10 , DOI: 10.1016/j.ejmech.2024.117156 Haibo Huo, Wenjia Dan, Min Li, Yanbin Chen, Chaofu Yang, Lintao Wu, Baojun Shi, Jian Li
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-10 , DOI: 10.1016/j.ejmech.2024.117156 Haibo Huo, Wenjia Dan, Min Li, Yanbin Chen, Chaofu Yang, Lintao Wu, Baojun Shi, Jian Li
|
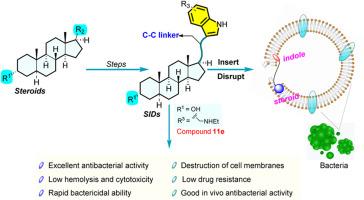
Rational modification of natural products plays a key role in drug discovery. Herein, a series of steroidal indole derivatives containing various substituents and steroidal skeletons were designed and synthesized with classical Fischer indole synthesis as a key step in an efficient synthetic route for the first time. The in vitro antibacterial activity of all the synthesized derivatives was evaluated against four Gram-positive strains including three Methicillin-Resistant Staphylococcus aureus . Compound 11e displayed the most potent antibacterial activity (MIC = 1–2 μg/mL) with low cytotoxicity and hemolytic activity. Derivative 11e displayed more rapid bactericidal kinetic than vancomycin in the time-kill study and was less likely to induce bacterial resistance. Moreover, the preliminary antibacterial mechanism explorations indicated that compound 11e could effectively inhibit biofilm formation, promote the accumulation of reactive oxygen species, decrease bacterial metabolism, and destroy bacterial cell membranes to exert its antibacterial effects. The study of in vivo antibacterial activity suggested that compound 11e could significantly reduce the bacteria counts in a mouse subcutaneous infection model. These findings provided a bright hope for steroidal indole derivatives as promising antibacterial candidates to settle drug resistance.
中文翻译:

甾体吲哚衍生物作为膜靶向抗菌候选物的设计、合成和生物学评价
天然产物的合理修饰在药物发现中起着关键作用。在此,设计了一系列包含各种取代基和甾体骨架的甾体吲哚衍生物,并首次用经典的 Fischer 吲哚合成作为高效合成路线的关键步骤。针对 4 种革兰氏阳性菌株(包括 3 种耐甲氧西林金黄色葡萄球菌)评价了所有合成衍生物的体外抗菌活性。化合物 11e 显示出最有效的抗菌活性 (MIC = 1–2 μg/mL),具有低细胞毒性和溶血活性。在时间杀灭研究中,衍生物 11e 比万古霉素显示出更快的杀菌动力学,并且不太可能诱导细菌耐药性。此外,初步的抗菌机制探索表明,化合物 11e 可有效抑制生物膜形成,促进活性氧的积累,减少细菌代谢,破坏细菌细胞膜以发挥其抗菌作用。体内抗菌活性研究表明,化合物 11e 可以显着降低小鼠皮下感染模型中的细菌数量。这些发现为甾体吲哚衍生物作为解决耐药性的有前途的抗菌候选物提供了光明的希望。
更新日期:2024-12-10
中文翻译:

甾体吲哚衍生物作为膜靶向抗菌候选物的设计、合成和生物学评价
天然产物的合理修饰在药物发现中起着关键作用。在此,设计了一系列包含各种取代基和甾体骨架的甾体吲哚衍生物,并首次用经典的 Fischer 吲哚合成作为高效合成路线的关键步骤。针对 4 种革兰氏阳性菌株(包括 3 种耐甲氧西林金黄色葡萄球菌)评价了所有合成衍生物的体外抗菌活性。化合物 11e 显示出最有效的抗菌活性 (MIC = 1–2 μg/mL),具有低细胞毒性和溶血活性。在时间杀灭研究中,衍生物 11e 比万古霉素显示出更快的杀菌动力学,并且不太可能诱导细菌耐药性。此外,初步的抗菌机制探索表明,化合物 11e 可有效抑制生物膜形成,促进活性氧的积累,减少细菌代谢,破坏细菌细胞膜以发挥其抗菌作用。体内抗菌活性研究表明,化合物 11e 可以显着降低小鼠皮下感染模型中的细菌数量。这些发现为甾体吲哚衍生物作为解决耐药性的有前途的抗菌候选物提供了光明的希望。






























 京公网安备 11010802027423号
京公网安备 11010802027423号