当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational studies on Mn(III)-catalyzed cyclopropanols: a case of Mn(III)-based metalloradical catalysis involving an α-Mn(II)-bound-alkyl radical key intermediate
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-11 , DOI: 10.1039/d4qo01775g Pan Hong, Xiu-Yuan Zou, Peng-Cheng Zhang, Xin Lu, Long-Wu Ye, Yi-Dong Luo, Qing Sun
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-11 , DOI: 10.1039/d4qo01775g Pan Hong, Xiu-Yuan Zou, Peng-Cheng Zhang, Xin Lu, Long-Wu Ye, Yi-Dong Luo, Qing Sun
|
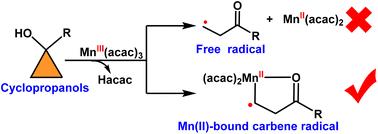
Controlling the reactivity of radicals has long posed a significant challenge in the field of radical chemistry. A groundbreaking strategy to address this challenge involves the use of metal-entangled organic radicals via metalloradical catalysis (MRC). Despite achievements in this domain, the substrates capable of generating metal-bound radical species have predominantly been of an oxidative nature, with scarce reports on reductive substrates. Herein, using DFT calculations, we present a novel finding: a reducing substrate, cyclopropanol, capable of generating metal-bound radicals via a Mn(acac)3 catalyst. Through detailed mechanistic exploration, we have determined that the free radical mechanism proposed in Mn(III)-catalyzed cyclopropanol reactions is energetically unfavorable, while the metal-bound radical mechanism can proceed smoothly at room temperature. Notably, the Mn(II)-bound carbene radical intermediate generated from cyclopropanol holds substantial potential for rational control over the diversity of radical reactions. Furthermore, the presence of metal-bound radical intermediates offers significant feasibility for designing stereoselective products by introducing auxiliary ligands in reactions involving cyclopropanols. This discovery highlights the potential of reducing substrates in metal-catalyzed radical reactions and unlocks new prospects for the development of redox-neutral radical catalytic processes. The ability to form well-defined metal-bound radicals from cyclopropanol not only expands the scope of substrates available for such transformations but also paves the way for advanced applications in stereoselective synthesis, leveraging the unique properties of metal coordination environments.
中文翻译:

Mn(III) 催化的环丙醇的计算研究:涉及 α-Mn(II) 结合烷基自由基关键中间体的基于 Mn(III) 的金属自由基催化案例
长期以来,控制自由基的反应性一直是自由基化学领域的重大挑战。应对这一挑战的开创性策略涉及通过金属自由基催化 (MRC) 使用金属纠缠有机自由基。尽管在这一领域取得了成就,但能够产生金属结合自由基物质的底物主要是氧化性的,关于还原底物的报道很少。在此,使用 DFT 计算,我们提出了一个新的发现:一种还原底物环丙醇,能够通过 Mn(acac)3 催化剂生成金属结合自由基。通过详细的机理探索,我们确定了 Mn(III) 催化的环丙醇反应中提出的自由基机理在能量上是不利的,而金属结合的自由基机理在室温下可以顺利进行。值得注意的是,由环丙醇生成的 Mn(II) 结合卡宾自由基中间体具有合理控制自由基反应多样性的巨大潜力。此外,金属结合自由基中间体的存在为通过在涉及环丙醇的反应中引入辅助配体来设计立体选择性产物提供了重要的可行性。这一发现突出了还原底物在金属催化自由基反应中的潜力,并为氧化还原中性自由基催化过程的发展开辟了新的前景。 从环丙醇形成定义明确的金属结合自由基的能力不仅扩大了可用于此类转化的底物的范围,而且还利用金属配位环境的独特特性,为立体选择性合成中的高级应用铺平了道路。
更新日期:2024-12-11
中文翻译:

Mn(III) 催化的环丙醇的计算研究:涉及 α-Mn(II) 结合烷基自由基关键中间体的基于 Mn(III) 的金属自由基催化案例
长期以来,控制自由基的反应性一直是自由基化学领域的重大挑战。应对这一挑战的开创性策略涉及通过金属自由基催化 (MRC) 使用金属纠缠有机自由基。尽管在这一领域取得了成就,但能够产生金属结合自由基物质的底物主要是氧化性的,关于还原底物的报道很少。在此,使用 DFT 计算,我们提出了一个新的发现:一种还原底物环丙醇,能够通过 Mn(acac)3 催化剂生成金属结合自由基。通过详细的机理探索,我们确定了 Mn(III) 催化的环丙醇反应中提出的自由基机理在能量上是不利的,而金属结合的自由基机理在室温下可以顺利进行。值得注意的是,由环丙醇生成的 Mn(II) 结合卡宾自由基中间体具有合理控制自由基反应多样性的巨大潜力。此外,金属结合自由基中间体的存在为通过在涉及环丙醇的反应中引入辅助配体来设计立体选择性产物提供了重要的可行性。这一发现突出了还原底物在金属催化自由基反应中的潜力,并为氧化还原中性自由基催化过程的发展开辟了新的前景。 从环丙醇形成定义明确的金属结合自由基的能力不仅扩大了可用于此类转化的底物的范围,而且还利用金属配位环境的独特特性,为立体选择性合成中的高级应用铺平了道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号