当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A novel method for enhancing vanadium extraction from calcified vanadium slag: H2SO4–Na2SO3 synergistic reduction leaching and short process preparation of vanadium dioxide
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-12-11 , DOI: 10.1039/d4ta07487d Changqing Li, Tao Jiang, Jing Wen, Guangdong Yang, Tangxia Yu, Lan Zhang
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-12-11 , DOI: 10.1039/d4ta07487d Changqing Li, Tao Jiang, Jing Wen, Guangdong Yang, Tangxia Yu, Lan Zhang
|
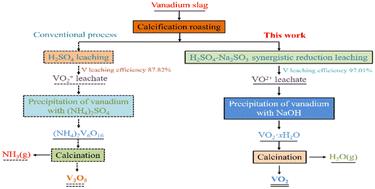
The primary shortcomings of the current vanadium extraction from the calcification vanadium slag process are low vanadium leaching efficiency, potential ammonia pollution risk, and the inability to directly obtain low-valence vanadium products. This research presents an innovative H2SO4–Na2SO3 synergistically enhanced reduction leaching method that has successfully achieved high-efficiency vanadium extraction. The results indicate that the introduction of Na2SO3 enhances the thermodynamic driving force of the acid leaching reaction under low-acidity conditions, makes the reduction of poorly soluble VO2+ to more soluble VO2+, promotes the acid leaching reaction, and lays the foundation for the preparation of VO2 by direct green hydrolysis and precipitation of vanadium from the leachate. Reducing the pH of the leaching system and increasing the Na2SO3 dosage are key to improving vanadium leaching efficiency. At a leaching pH of 1.8, with a Na2SO3 excess coefficient of 1.5, a leaching duration of 60 min, a liquid-to-solid ratio of 10 : 1 mL g−1, and a leaching temperature of 90 °C, the vanadium leaching efficiency reached 97.01%, reflecting a 10.23% improvement compared to conventional H2SO4 leaching. During the hydrolytic precipitation of VO2+, a lower precipitation pH enhances the separation of vanadium from impurities in the leachate. Under optimal conditions, precipitation pH of 5, precipitation time of 10 min, and a temperature of 25 °C, VO2·xH2O was obtained with a precipitation efficiency of 96.11%. After washing and calcination, VO2 was obtained with a purity of 98.49%. This innovative reduction leaching process enables efficient vanadium extraction and a streamlined production of low-valence vanadium compounds, while circumventing the environmental concerns associated with conventional ammonium salt precipitation methods.
中文翻译:

一种强化钙化钒渣提钒的新方法:H2SO4–Na2SO3协同还原浸出及二氧化钒的短工艺制备
目前从钙化钒渣工艺中提取钒的主要缺点是钒浸出效率低、潜在的氨污染风险以及无法直接获得低价钒产品。本研究提出了一种创新的 H2SO4-Na 2SO3 协同增强还原浸出方法,该方法已成功实现高效钒萃取。结果表明,Na2SO3 的引入增强了低酸性条件下酸浸反应的热力学驱动力,使难溶性 VO2+ 还原为更易溶的 VO2+,促进了酸浸反应,为制备 VO2 奠定了基础通过直接绿色水解并从渗滤液中沉淀钒。降低浸出系统的 pH 值和增加 Na2SO3 的用量是提高钒浸出效率的关键。在浸出 pH 值为 1.8、Na2SO3 过量系数为 1.5、浸出持续时间为 60 分钟、液固比为 10 : 1 mL g-1 和浸出温度为 90 °C 时,钒浸出效率达到 97.01%,与传统的 H2SO4 浸出相比提高了 10.23%。在 VO2+ 的水解沉淀过程中,较低的沉淀 pH 值可增强钒与渗滤液中杂质的分离。 在最佳条件下,沉淀pH为5,沉淀时间为10 min,温度为25 °C,VO2·倍得到 H2O,沉淀效率为 96.11%。经洗涤和煅烧,得到 VO2,纯度为 98.49%。这种创新的还原浸出工艺可实现高效的钒提取和低价钒化合物的简化生产,同时规避了与传统铵盐沉淀方法相关的环境问题。
更新日期:2024-12-11
中文翻译:

一种强化钙化钒渣提钒的新方法:H2SO4–Na2SO3协同还原浸出及二氧化钒的短工艺制备
目前从钙化钒渣工艺中提取钒的主要缺点是钒浸出效率低、潜在的氨污染风险以及无法直接获得低价钒产品。本研究提出了一种创新的 H2SO4-Na 2SO3 协同增强还原浸出方法,该方法已成功实现高效钒萃取。结果表明,Na2SO3 的引入增强了低酸性条件下酸浸反应的热力学驱动力,使难溶性 VO2+ 还原为更易溶的 VO2+,促进了酸浸反应,为制备 VO2 奠定了基础通过直接绿色水解并从渗滤液中沉淀钒。降低浸出系统的 pH 值和增加 Na2SO3 的用量是提高钒浸出效率的关键。在浸出 pH 值为 1.8、Na2SO3 过量系数为 1.5、浸出持续时间为 60 分钟、液固比为 10 : 1 mL g-1 和浸出温度为 90 °C 时,钒浸出效率达到 97.01%,与传统的 H2SO4 浸出相比提高了 10.23%。在 VO2+ 的水解沉淀过程中,较低的沉淀 pH 值可增强钒与渗滤液中杂质的分离。 在最佳条件下,沉淀pH为5,沉淀时间为10 min,温度为25 °C,VO2·倍得到 H2O,沉淀效率为 96.11%。经洗涤和煅烧,得到 VO2,纯度为 98.49%。这种创新的还原浸出工艺可实现高效的钒提取和低价钒化合物的简化生产,同时规避了与传统铵盐沉淀方法相关的环境问题。






























 京公网安备 11010802027423号
京公网安备 11010802027423号