Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Automated fibril structure calculations in Xplor-NIH
Structure ( IF 4.4 ) Pub Date : 2024-12-10 , DOI: 10.1016/j.str.2024.11.011 Alexander M. Barclay, Moses H. Milchberg, Owen A. Warmuth, Marcus D. Tuttle, Christopher J. Dennis, Charles D. Schwieters, Chad M. Rienstra
Structure ( IF 4.4 ) Pub Date : 2024-12-10 , DOI: 10.1016/j.str.2024.11.011 Alexander M. Barclay, Moses H. Milchberg, Owen A. Warmuth, Marcus D. Tuttle, Christopher J. Dennis, Charles D. Schwieters, Chad M. Rienstra
|
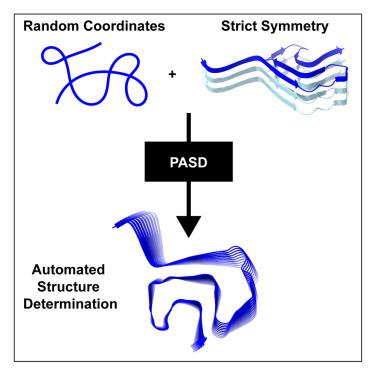
Amyloid fibrils are protein assemblies that are pathologically linked to neurodegenerative diseases. Fibril structures can aid development of highly specific ligands for diagnostic imaging and therapeutics. Solid-state NMR (SSNMR) is a viable approach to solving fibril structures; however, most SSNMR protocols require manual analysis of extensive spectral data, presenting a major bottleneck to determining structures. Standard automation; routines fall short for symmetric multimeric assemblies like amyloids due to high cross peak degeneracy and the need to account for multiple protein subunits. Here, we employ the probabilistic assignment for structure determination protocol in conjunction with strict; symmetry in Xplor-NIH structure determination software, demonstrating the methodology using data from a previous structure of an α-synuclein (Asyn) fibril implicated in Parkinson disease. The automated protocol generated a structure of comparable, if not superior, quality in a few days of computational time, reducing the manual effort required; to solve amyloid structures by SSNMR.
中文翻译:

Xplor-NIH 中的自动原纤维结构计算
淀粉样蛋白原纤维是与神经退行性疾病在病理学上相关的蛋白质组装体。原纤维结构可以帮助开发用于诊断成像和治疗的高度特异性配体。固体 NMR (SSNMR) 是解决原纤维结构的可行方法;然而,大多数 SSNMR 协议需要手动分析大量的光谱数据,这是确定结构的主要瓶颈。标准自动化;由于高交叉峰简并性并且需要考虑多个蛋白质亚基,因此对于对称多聚体组装(如淀粉样蛋白)来说,常规操作达不到要求。在这里,我们将结构确定协议的概率分配与 strict 结合使用;Xplor-NIH 结构测定软件中的对称性,使用与帕金森病相关的 α-突触核蛋白 (Asyn) 原纤维的先前结构数据展示了该方法。自动化方案在几天的计算时间内生成了一个质量相当(如果不是更优越)的结构,从而减少了所需的手动工作;通过 SSNMR 解决淀粉样蛋白结构。
更新日期:2024-12-10
中文翻译:

Xplor-NIH 中的自动原纤维结构计算
淀粉样蛋白原纤维是与神经退行性疾病在病理学上相关的蛋白质组装体。原纤维结构可以帮助开发用于诊断成像和治疗的高度特异性配体。固体 NMR (SSNMR) 是解决原纤维结构的可行方法;然而,大多数 SSNMR 协议需要手动分析大量的光谱数据,这是确定结构的主要瓶颈。标准自动化;由于高交叉峰简并性并且需要考虑多个蛋白质亚基,因此对于对称多聚体组装(如淀粉样蛋白)来说,常规操作达不到要求。在这里,我们将结构确定协议的概率分配与 strict 结合使用;Xplor-NIH 结构测定软件中的对称性,使用与帕金森病相关的 α-突触核蛋白 (Asyn) 原纤维的先前结构数据展示了该方法。自动化方案在几天的计算时间内生成了一个质量相当(如果不是更优越)的结构,从而减少了所需的手动工作;通过 SSNMR 解决淀粉样蛋白结构。

































 京公网安备 11010802027423号
京公网安备 11010802027423号