当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gas hydrate conditions prediction in the presence of salts by considering modified relative permittivity of solution; Application of the Kirkwood-Onsager approach
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2024-12-10 , DOI: 10.1016/j.ces.2024.121060 Tianwen Luo
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2024-12-10 , DOI: 10.1016/j.ces.2024.121060 Tianwen Luo
|
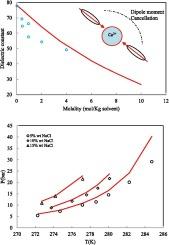
This work aims to study the effect of the relative permittivity of electrolyte solution on hydrate dissociation pressure prediction. Accurate prediction of the water activity plays the main role in the prediction of hydrate dissociation conditions in electrolyte containing systems. The relative permittivity of aqueous electrolyte solution is temperature and concentration-dependent. Therefore, the effect of salt concentration and temperature on the relative permittivity of aqueous electrolyte solutions must be considered to predict the hydrate formation condition accurately. In this study, the modified permittivity of aqueous electrolyte solution has been obtained using the modified Kirkwood g-factor. The electrolyte PC-SAFT EoS has been utilized to calculate the gas fugacity and water activity in the phase equilibrium calculation of hydrate formation. The van der Waals and Platteeuw model has been utilized to predict the hydrate formation condition of methane, ethane, and propane in the presence of NaCl, KCl, and CaCl2 electrolyte solutions over a wide range of salt concentrations and temperatures. The model results have been compared with those obtained using a constant or concentration-independent dielectric constant. The results show that the effect of salt concentration on the relative permittivity of aqueous electrolyte solution must be considered. The proposed methodology improves the thermodynamic model performance dramatically. The aforementioned approach can be utilized for all EoS-based models to predict the hydrate dissociation conditions in the presence of electrolytes.
中文翻译:

通过考虑溶液的修正相对介电常数,在盐存在下预测天然气水合物条件;Kirkwood-Onsager 方法的应用
本研究旨在研究电解质溶液的相对介电常数对水合物解离压力预测的影响。在含电解质系统中水合物解离条件的预测中,水活度的准确预测起着主要作用。电解质水溶液的相对介电常数与温度和浓度有关。因此,必须考虑盐浓度和温度对电解质水溶液相对介电常数的影响,以准确预测水合物的形成条件。在本研究中,使用改进的 Kirkwood g 因子获得了电解质水溶液的修正介电常数。电解质 PC-SAFT EoS 已用于计算水合物形成的相平衡计算中的气体逸度和水活度。van der Waals 和 Platteeuw 模型已被用于预测在很宽的盐浓度和温度范围内,在 NaCl、KCl 和 CaCl2 电解质溶液存在下甲烷、乙烷和丙烷的水合物形成条件。模型结果已与使用常数或浓度无关的介电常数获得的结果进行了比较。结果表明,必须考虑盐浓度对电解质水溶液相对介电常数的影响。所提出的方法显著提高了热力学模型的性能。上述方法可用于所有基于 EoS 的模型,以预测存在电解质下的水合物解离条件。
更新日期:2024-12-10
中文翻译:

通过考虑溶液的修正相对介电常数,在盐存在下预测天然气水合物条件;Kirkwood-Onsager 方法的应用
本研究旨在研究电解质溶液的相对介电常数对水合物解离压力预测的影响。在含电解质系统中水合物解离条件的预测中,水活度的准确预测起着主要作用。电解质水溶液的相对介电常数与温度和浓度有关。因此,必须考虑盐浓度和温度对电解质水溶液相对介电常数的影响,以准确预测水合物的形成条件。在本研究中,使用改进的 Kirkwood g 因子获得了电解质水溶液的修正介电常数。电解质 PC-SAFT EoS 已用于计算水合物形成的相平衡计算中的气体逸度和水活度。van der Waals 和 Platteeuw 模型已被用于预测在很宽的盐浓度和温度范围内,在 NaCl、KCl 和 CaCl2 电解质溶液存在下甲烷、乙烷和丙烷的水合物形成条件。模型结果已与使用常数或浓度无关的介电常数获得的结果进行了比较。结果表明,必须考虑盐浓度对电解质水溶液相对介电常数的影响。所提出的方法显著提高了热力学模型的性能。上述方法可用于所有基于 EoS 的模型,以预测存在电解质下的水合物解离条件。






























 京公网安备 11010802027423号
京公网安备 11010802027423号