当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
VUV photochemistry of cyclopropenone (c-C3H2O): formation rate and abundance ratios of propynal (HCCCHO) and propadienone (CH2CCO)
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-10 , DOI: 10.1039/d4cp03895a Mohamad Ibrahim, Jean-Claude Guillemin, Lahouari Krim
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-10 , DOI: 10.1039/d4cp03895a Mohamad Ibrahim, Jean-Claude Guillemin, Lahouari Krim
|
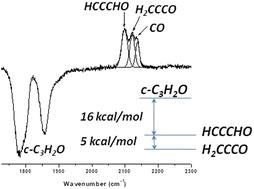
The distribution of isomeric species in the interstellar medium cannot be directly related to their relative energetic stabilities but more to their mechanisms of formation and evolution. The abundances of the three isomers of C3H2O, cyclopropenone, propynal and propadienone, are an example among many other interstellar species wherein kinetic effects control their presence in astrophysical regions. To date, only propynal and cyclopropenone, the two less stable isomers of propadienone, have been detected in the interstellar medium. In this work, we examined the vacuum ultraviolet (VUV) photochemistry of cyclopropenone (c-C3H2O), an unsaturated ketone and aromatic compound, which is the most thermodynamically unstable form of the three isomers of C3H2O. Using Fourier transform infrared spectroscopy and mass spectrometry to probe the photoproducts, we investigated the photolysis of cyclopropenone ice and cyclopropenone trapped in a neon matrix. We showed that the VUV photolysis of c-C3H2O ice at 10 K led mainly to CO and C2H2 fragments, in addition to propynal HCCCHO and propadienone CH2CCO. The distribution of the two isomers depended on the irradiation time. Within a short irradiation time, HCCCHO was formed in twice the abundance of the most stable isomer CH2CCO, while upon increasing the irradiation time, the same abundances were observed, showing that equilibrium was reached between the two isomers. The photolysis of c-C3H2O isolated in the neon matrix allowed the characterization of HCCCO and CCCO as reaction intermediates, resulting from the VUV-induced dehydrogenation of cyclopropenone, which are the precursors of propynal and propadienone through the CCCO + 2H reaction.
中文翻译:

环丙烯酮 (c-C3H2O) 的 VUV 光化学:丙醛 (HCCCHO) 和丙二烯酮 (CH2CCO) 的形成速率和丰度比
异构体物质在星际介质中的分布不能直接与它们的相对能量稳定性相关,而更多地与它们的形成和演化机制有关。C3H2O 的三种异构体、环丙烯酮、丙炔醛和丙二烯酮的丰度在许多其他星际物种中显示了一个例子,其中动力学效应会控制它们在天体物理区域的存在。直到今天,在星际介质中只检测到丙炔酮和环丙烯酮这两种不太稳定的异构体。在这项工作中,我们研究了环丙烯酮 (c-C3H2O) 的真空紫外 (VUV) 光化学,环丙烯酮是一种不饱和酮和芳香族化合物,是 C3H2O 的三种异构体中热力学上最不稳定的形式。使用傅里叶变换红外光谱和质谱探测光产物,我们研究了被困在氖基体中的环丙烯酮冰和环丙烯酮的光解。我们表明,除了丙炔醛 HCCCHO 和丙二烯酮 CH2CCO 外,c-C3H2O 冰在 10 K 下的 VUV 光解主要导致 CO 和 C2H2 片段。两种异构体的分布取决于照射时间。在较短的照射时间内,HCCCO 的丰度是最稳定的异构体 CH2CCO 的两倍,而通过增加照射时间,观察到相同的丰度,表明两种异构体之间达到平衡。在氖基体中分离的 c-C3H2O 的光解使得 HCCCO 和 CCCO 作为反应中间体的表征,这些反应中间体是由 VUV 诱导的环丙烯酮脱氢产生的,它们是丙炔醛和丙二烯酮通过 CCCO + 2H 反应的前体。
更新日期:2024-12-10
中文翻译:

环丙烯酮 (c-C3H2O) 的 VUV 光化学:丙醛 (HCCCHO) 和丙二烯酮 (CH2CCO) 的形成速率和丰度比
异构体物质在星际介质中的分布不能直接与它们的相对能量稳定性相关,而更多地与它们的形成和演化机制有关。C3H2O 的三种异构体、环丙烯酮、丙炔醛和丙二烯酮的丰度在许多其他星际物种中显示了一个例子,其中动力学效应会控制它们在天体物理区域的存在。直到今天,在星际介质中只检测到丙炔酮和环丙烯酮这两种不太稳定的异构体。在这项工作中,我们研究了环丙烯酮 (c-C3H2O) 的真空紫外 (VUV) 光化学,环丙烯酮是一种不饱和酮和芳香族化合物,是 C3H2O 的三种异构体中热力学上最不稳定的形式。使用傅里叶变换红外光谱和质谱探测光产物,我们研究了被困在氖基体中的环丙烯酮冰和环丙烯酮的光解。我们表明,除了丙炔醛 HCCCHO 和丙二烯酮 CH2CCO 外,c-C3H2O 冰在 10 K 下的 VUV 光解主要导致 CO 和 C2H2 片段。两种异构体的分布取决于照射时间。在较短的照射时间内,HCCCO 的丰度是最稳定的异构体 CH2CCO 的两倍,而通过增加照射时间,观察到相同的丰度,表明两种异构体之间达到平衡。在氖基体中分离的 c-C3H2O 的光解使得 HCCCO 和 CCCO 作为反应中间体的表征,这些反应中间体是由 VUV 诱导的环丙烯酮脱氢产生的,它们是丙炔醛和丙二烯酮通过 CCCO + 2H 反应的前体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号