当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
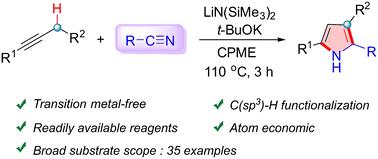
Synthesis of 2,3,5-trisubstituted 1H-pyrroles via formal [3 + 2] cycloaddition of 1-arylpropynes and nitriles and their antiproliferative activities
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-10 , DOI: 10.1039/d4qo01999g Dandan He, Ling Li, Xiong Wen, Luxiang Yin, Jue Li, Sha Wu, Huili Li, Fei Jiang, Xiangchun Shen
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-10 , DOI: 10.1039/d4qo01999g Dandan He, Ling Li, Xiong Wen, Luxiang Yin, Jue Li, Sha Wu, Huili Li, Fei Jiang, Xiangchun Shen
|
A straightforward approach for the assembly of 2,3,5-trisubstituted 1H-pyrroles via a base-mediated [3 + 2] cycloaddition of 1-arylpropynes with nitriles has been reported through alkali metal salt-promoted C(sp3)–H functionalization. This reaction features a transition metal-free catalyst, facile starting materials and a wide substrate scope. Moreover, the biological evaluation revealed that these pyrrole products exhibit antiproliferative activities against MDA-MB-231, SGC-7901, and HCT-116 cells, which provides potential applications in pharmaceutical chemistry.
中文翻译:

通过 1-芳基丙炔和腈的正式 [3 + 2] 环加成反应合成 2,3,5-三取代的 1H-吡咯及其抗增殖活性
据报道,通过碱金属盐促进的 C(sp3)-H 官能化,通过碱介导的 1-芳基丙炔与腈的 [3 + 2] 环加成反应组装 2,3,5-三取代 1-H-吡咯的一种直接方法。该反应具有无过渡金属催化剂、简单的起始材料和广泛的底物范围。此外,生物学评价显示,这些吡咯产物对 MDA-MB-231、SGC-7901 和 HCT-116 细胞表现出抗增殖活性,这在药物化学中提供了潜在的应用。
更新日期:2024-12-10
中文翻译:

通过 1-芳基丙炔和腈的正式 [3 + 2] 环加成反应合成 2,3,5-三取代的 1H-吡咯及其抗增殖活性
据报道,通过碱金属盐促进的 C(sp3)-H 官能化,通过碱介导的 1-芳基丙炔与腈的 [3 + 2] 环加成反应组装 2,3,5-三取代 1-H-吡咯的一种直接方法。该反应具有无过渡金属催化剂、简单的起始材料和广泛的底物范围。此外,生物学评价显示,这些吡咯产物对 MDA-MB-231、SGC-7901 和 HCT-116 细胞表现出抗增殖活性,这在药物化学中提供了潜在的应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号