当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-symmetric cysteine stapling in native peptides and proteins
Chemical Communications ( IF 4.3 ) Pub Date : 2024-12-10 , DOI: 10.1039/d4cc04995k Sven Ullrich, Bishvanwesha Panda, Upamali Somathilake, Douglas J. Lawes, Christoph Nitsche
Chemical Communications ( IF 4.3 ) Pub Date : 2024-12-10 , DOI: 10.1039/d4cc04995k Sven Ullrich, Bishvanwesha Panda, Upamali Somathilake, Douglas J. Lawes, Christoph Nitsche
|
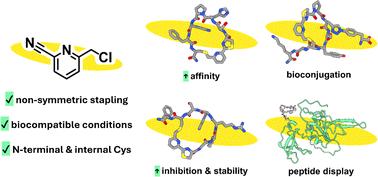
Stapling rigidifies peptides through covalent linkages between amino acids. We introduce 2-chloromethyl-6-cyanopyridine for non-symmetric stapling of N-terminal and internal cysteines. This biocompatible method produces diverse peptide macrocycles with enhanced affinity, stability and inhibitory potency. It is applicable to native peptides and proteins alike, demonstrating potential for peptide drug discovery platforms.
中文翻译:

天然肽和蛋白质中的非对称半胱氨酸吻合
装订通过氨基酸之间的共价键使肽刚性化。我们引入了 2-氯甲基-6-氰基吡啶,用于 N 端和内部半胱氨酸的非对称吻合。这种生物相容性方法可产生多种肽大环,具有增强的亲和力、稳定性和抑制效力。它适用于天然肽和蛋白质,展示了肽药物发现平台的潜力。
更新日期:2024-12-10
中文翻译:

天然肽和蛋白质中的非对称半胱氨酸吻合
装订通过氨基酸之间的共价键使肽刚性化。我们引入了 2-氯甲基-6-氰基吡啶,用于 N 端和内部半胱氨酸的非对称吻合。这种生物相容性方法可产生多种肽大环,具有增强的亲和力、稳定性和抑制效力。它适用于天然肽和蛋白质,展示了肽药物发现平台的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号