当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two BN Isosteres of Anthracene: Synthesis and Characterization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-10-14 , DOI: 10.1021/ja508813v Jacob S. A. Ishibashi 1, 2 , Jonathan L. Marshall 2 , Audrey Mazière 3 , Gabriel J. Lovinger 1, 2 , Bo Li 1 , Lev N. Zakharov 2 , Alain Dargelos 3 , Alain Graciaa 3 , Anna Chrostowska 3 , Shih-Yuan Liu 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-10-14 , DOI: 10.1021/ja508813v Jacob S. A. Ishibashi 1, 2 , Jonathan L. Marshall 2 , Audrey Mazière 3 , Gabriel J. Lovinger 1, 2 , Bo Li 1 , Lev N. Zakharov 2 , Alain Dargelos 3 , Alain Graciaa 3 , Anna Chrostowska 3 , Shih-Yuan Liu 1, 2
Affiliation
|
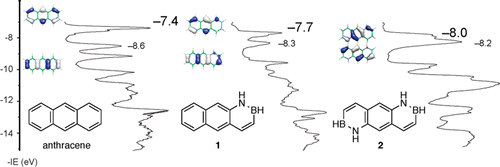
The synthesis of two parental BN anthracenes, 1 and 2, was developed, and their electronic structure and reactivity behavior were characterized in direct comparison with all-carbon anthracene. Gas-phase UV-photoelecton spectroscopy studies revealed the following HOMO energy trend: anthracene, -7.4 eV; BN anthracene 1, -7.7 eV; bis-BN anthracene 2, -8.0 eV. The λmax of the lower energy band in the UV-vis absorption spectrum is as follows: anthracene, 356 nm; BN anthracene 1, 359 nm; bis-BN anthracene 2, 357 nm. Thus, although the HOMO is stabilized with increasing BN incorporation, the HOMO-LUMO band gap remains unchanged across the anthracene series. The emission λmax values for the three investigated anthracene compounds are at 403 nm. The pKa values of the N-H proton for BN anthracene 1 and bis-BN anthracene 2 were determined to be approximately 26. BN anthracenes 1 and 2 do not undergo heat- or light-induced cycloaddition reactions or Friedel-Crafts acylations. Electrophilic bromination of BN anthracene 1 with Br2, however, occurs regioselectively at the 9-position. The reactivity behavior and regioselectivity of bromination of BN anthracenes are consistent with the electronic structure of these compounds; i.e., (1) the lower HOMO energy levels for BN anthracenes stabilize the molecules against cycloaddition and Friedel-Crafts reactions, and (2) the HOMO orbital coefficients are consistent with the observed bromination regioselectivity. Overall, this work demonstrates that BN/CC isosterism can be used as a molecular design strategy to stabilize the HOMO of acene-type structures while the optical band gap is maintained.
中文翻译:

蒽的两种 BN 等排体:合成和表征
开发了两种母体 BN 蒽 1 和 2 的合成,并与全碳蒽直接比较,表征了它们的电子结构和反应行为。气相紫外光电子能谱研究揭示了以下 HOMO 能量趋势:蒽,-7.4 eV;BN 蒽 1,-7.7 eV;双 BN 蒽 2,-8.0 eV。UV-vis吸收光谱中低能带的λmax为:蒽,356nm;BN 蒽 1, 359 nm; 双 BN 蒽 2, 357 nm。因此,尽管 HOMO 随着 BN 掺入的增加而稳定,但 HOMO-LUMO 带隙在整个蒽系列中保持不变。三种研究的蒽化合物的发射 λmax 值为 403 nm。BN 蒽 1 和双 BN 蒽 2 的 NH 质子的 pKa 值被确定为大约 26。BN 蒽 1 和 2 不经历热或光诱导的环加成反应或 Friedel-Crafts 酰化。然而,BN 蒽 1 与 Br2 的亲电溴化在 9 位发生区域选择性。BN蒽溴化反应的反应行为和区域选择性与这些化合物的电子结构一致;即,(1) BN 蒽较低的 HOMO 能级使分子稳定,防止环加成和 Friedel-Crafts 反应,(2) HOMO 轨道系数与观察到的溴化区域选择性一致。全面的,
更新日期:2014-10-14
中文翻译:

蒽的两种 BN 等排体:合成和表征
开发了两种母体 BN 蒽 1 和 2 的合成,并与全碳蒽直接比较,表征了它们的电子结构和反应行为。气相紫外光电子能谱研究揭示了以下 HOMO 能量趋势:蒽,-7.4 eV;BN 蒽 1,-7.7 eV;双 BN 蒽 2,-8.0 eV。UV-vis吸收光谱中低能带的λmax为:蒽,356nm;BN 蒽 1, 359 nm; 双 BN 蒽 2, 357 nm。因此,尽管 HOMO 随着 BN 掺入的增加而稳定,但 HOMO-LUMO 带隙在整个蒽系列中保持不变。三种研究的蒽化合物的发射 λmax 值为 403 nm。BN 蒽 1 和双 BN 蒽 2 的 NH 质子的 pKa 值被确定为大约 26。BN 蒽 1 和 2 不经历热或光诱导的环加成反应或 Friedel-Crafts 酰化。然而,BN 蒽 1 与 Br2 的亲电溴化在 9 位发生区域选择性。BN蒽溴化反应的反应行为和区域选择性与这些化合物的电子结构一致;即,(1) BN 蒽较低的 HOMO 能级使分子稳定,防止环加成和 Friedel-Crafts 反应,(2) HOMO 轨道系数与观察到的溴化区域选择性一致。全面的,































 京公网安备 11010802027423号
京公网安备 11010802027423号