Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low background electrochemical sensor based on HCR towards acute myocardial infarction-specific miRNA detection
Analyst ( IF 3.6 ) Pub Date : 2024-12-09 , DOI: 10.1039/d4an01065e Yan Liu, Ziqi Liu, Tingxiu Yan, Luyao Feng, Na He, Lu Tao, Li-Ping Xu, Xueji Zhang
Analyst ( IF 3.6 ) Pub Date : 2024-12-09 , DOI: 10.1039/d4an01065e Yan Liu, Ziqi Liu, Tingxiu Yan, Luyao Feng, Na He, Lu Tao, Li-Ping Xu, Xueji Zhang
|
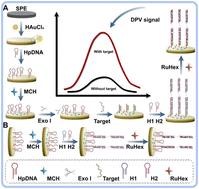
Acute myocardial infarction (AMI) accounts for a significant proportion of global fatalities, and early detection is crucial for improving patient outcomes. However, current diagnostic methods often struggle to detect AMI in its early stages. Herein, we present an electrochemical sensor utilizing a fractal gold (FracAu) electrode and hybridization chain reaction (HCR) amplification technology to detect AMI-specific microRNAs (miRNAs). When the target sequence was added, the HCR was triggered, leading to the formation of a long-nicked DNA double helix that efficiently captured a larger quantity of positively charged RuHex molecules, resulting in significant electrochemical signal amplification. More importantly, to avoid false positive signals, exonuclease I (Exo I) was introduced to selectively cleave single-stranded DNA (ssDNA) probes. These ssDNA probes, underwent random hydrolysis from hpDNA probes, could hybridize with helper DNA1 in the absence of the target, initiating the HCR process and producing a false positive signal. The inclusion of Exo I effectively avoided false positive signals and reduced background noise. Under optimized conditions, the fabricated sensor exhibited significant sensitivity and selectivity, showing a broad linear detection range from 10 pM to 10 nM and a low limit of 0.9 fM. The fabricated electrochemical sensor also successfully detected AMI-specific miRNA in real serum samples, underscoring its diagnostic promise. By providing a reliable tool for early detection, the innovative sensor holds significant potential in combating global cardiovascular disease-related mortality rates.
中文翻译:

基于 HCR 的低本底电化学传感器对急性心肌梗死特异性 miRNA 检测
急性心肌梗死 (AMI) 占全球死亡人数的很大一部分,早期发现对于改善患者预后至关重要。然而,目前的诊断方法往往难以在早期阶段检测到 AMI。在此,我们提出了一种利用分形金 (FracAu) 电极和杂交链反应 (HCR) 扩增技术来检测 AMI 特异性 microRNA (miRNA) 的电化学传感器。当添加靶序列时,HCR 被触发,导致形成长切口 DNA 双螺旋,该双螺旋有效捕获大量带正电荷的 RuHex 分子,从而显着放大电化学信号。更重要的是,为避免假阳性信号,引入了核酸外切酶 I (Exo I) 来选择性切割单链 DNA (ssDNA) 探针。这些 ssDNA 探针从 hpDNA 探针中随机水解,可以在没有靶标的情况下与辅助 DNA1 杂交,启动 HCR 过程并产生假阳性信号。Exo I 的加入有效地避免了假阳性信号并降低了背景噪音。在优化条件下,制造的传感器表现出显著的灵敏度和选择性,显示出 10 pM 至 10 nM 的宽线性检测范围和 0.9 fM 的下限。制造的电化学传感器还成功检测到真实血清样品中的 AMI 特异性 miRNA,凸显了其诊断前景。通过提供可靠的早期检测工具,这款创新传感器在对抗全球心血管疾病相关死亡率方面具有巨大潜力。
更新日期:2024-12-13
中文翻译:

基于 HCR 的低本底电化学传感器对急性心肌梗死特异性 miRNA 检测
急性心肌梗死 (AMI) 占全球死亡人数的很大一部分,早期发现对于改善患者预后至关重要。然而,目前的诊断方法往往难以在早期阶段检测到 AMI。在此,我们提出了一种利用分形金 (FracAu) 电极和杂交链反应 (HCR) 扩增技术来检测 AMI 特异性 microRNA (miRNA) 的电化学传感器。当添加靶序列时,HCR 被触发,导致形成长切口 DNA 双螺旋,该双螺旋有效捕获大量带正电荷的 RuHex 分子,从而显着放大电化学信号。更重要的是,为避免假阳性信号,引入了核酸外切酶 I (Exo I) 来选择性切割单链 DNA (ssDNA) 探针。这些 ssDNA 探针从 hpDNA 探针中随机水解,可以在没有靶标的情况下与辅助 DNA1 杂交,启动 HCR 过程并产生假阳性信号。Exo I 的加入有效地避免了假阳性信号并降低了背景噪音。在优化条件下,制造的传感器表现出显著的灵敏度和选择性,显示出 10 pM 至 10 nM 的宽线性检测范围和 0.9 fM 的下限。制造的电化学传感器还成功检测到真实血清样品中的 AMI 特异性 miRNA,凸显了其诊断前景。通过提供可靠的早期检测工具,这款创新传感器在对抗全球心血管疾病相关死亡率方面具有巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号