当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible-Deactivation Radical Polymerization of Vinyl Monomers Mediated by Schiff Bases
Macromolecules ( IF 5.1 ) Pub Date : 2024-12-08 , DOI: 10.1021/acs.macromol.4c02195 Wachara Benchaphanthawee, Zhe-Wei Yang, Huei-Ting Hsu, Chi-How Peng
Macromolecules ( IF 5.1 ) Pub Date : 2024-12-08 , DOI: 10.1021/acs.macromol.4c02195 Wachara Benchaphanthawee, Zhe-Wei Yang, Huei-Ting Hsu, Chi-How Peng
|
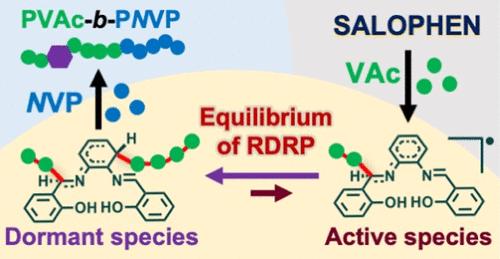
N,N′-Bis(salicylidene)-1,2-phenylenediamine (salophen), a versatile ligand for organometallics, was found to be capable of mediating the reversible-deactivation radical polymerization (RDRP) of vinyl acetate (VAc) and N-vinylpyrrolidone (NVP) with predictable molecular weights and the formation of block copolymers. The mechanism was first studied via the substituent effect with different salophen derivatives and then rationalized by density functional theory (DFT) calculations associated with the control studies and chain-end characterization. We discovered that the radicals react with the imine carbon of salophen to generate an active species that further deactivates the propagating radicals to form the dormant species. The observed living characteristics were attributed to the equilibrium between the dormant species and the active species with radicals.
中文翻译:

由 Schiff 碱介导的乙烯基单体的可逆失活自由基聚合
N,N′-双(水杨烯)-1,2-苯二胺(salophen)是一种用于有机金属化合物的多功能配体,被发现能够介导具有可预测分子量的醋酸乙烯酯 (VAc) 和 N-乙烯基吡咯烷酮 (NVP) 的可逆失活自由基聚合 (RDRP) 和嵌段共聚物的形成。首先通过不同 salophen 衍生物的取代基效应研究该机制,然后通过与对照研究和链端表征相关的密度泛函理论 (DFT) 计算进行合理化。我们发现自由基与所罗芬的亚胺碳反应产生一种活性物质,该物质进一步使繁殖的自由基失活,形成休眠物种。观察到的生活特性归因于休眠物种和具有自由基的活性物种之间的平衡。
更新日期:2024-12-09
中文翻译:

由 Schiff 碱介导的乙烯基单体的可逆失活自由基聚合
N,N′-双(水杨烯)-1,2-苯二胺(salophen)是一种用于有机金属化合物的多功能配体,被发现能够介导具有可预测分子量的醋酸乙烯酯 (VAc) 和 N-乙烯基吡咯烷酮 (NVP) 的可逆失活自由基聚合 (RDRP) 和嵌段共聚物的形成。首先通过不同 salophen 衍生物的取代基效应研究该机制,然后通过与对照研究和链端表征相关的密度泛函理论 (DFT) 计算进行合理化。我们发现自由基与所罗芬的亚胺碳反应产生一种活性物质,该物质进一步使繁殖的自由基失活,形成休眠物种。观察到的生活特性归因于休眠物种和具有自由基的活性物种之间的平衡。































 京公网安备 11010802027423号
京公网安备 11010802027423号