当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Singlet (1Δg) O2 initiated gas phase oxidation as a potential tropospheric decay channel for ketene
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-09 , DOI: 10.1039/d4cp04026k Saptarshi Sarkar, Ashray Dhiman, Biman Bandyopadhyay
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-09 , DOI: 10.1039/d4cp04026k Saptarshi Sarkar, Ashray Dhiman, Biman Bandyopadhyay
|
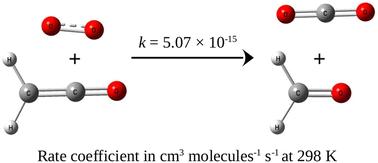
The oxidation of CH2CO by (1Δg) O2 has been investigated by means of high level quantum chemical and chemical kinetic calculations. The reaction was found to proceed through a four-membered cyclic transition state resulting from the addition of O2 to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of ketene. The reaction energetics has been calculated employing post-CCSD(T) corrections. The energy of the transition state was found to be 33.6 kcal mol−1 below that of the isolated reactants. The rate coefficient, calculated using master equations under tropospheric conditions, was found to be 5.1 × 10−15 cm3 molecule−1 s−1 at 298 K and 1 bar. Atmospheric implications of the title reaction have been estimated by comparing the atmospheric lifetime of ketene for the title reaction against reactions with ˙OH, H2O and NH3. On a global scale, the lifetime for the title reaction was found to be almost 70 times that for the reaction with ˙OH. However, under special conditions, where the local concentration of singlet O2 is significantly higher and/or the concentration of ˙OH is significantly lower, singlet O2 initiated oxidation could become the most significant tropospheric loss mechanism of CH2CO.
C bond of ketene. The reaction energetics has been calculated employing post-CCSD(T) corrections. The energy of the transition state was found to be 33.6 kcal mol−1 below that of the isolated reactants. The rate coefficient, calculated using master equations under tropospheric conditions, was found to be 5.1 × 10−15 cm3 molecule−1 s−1 at 298 K and 1 bar. Atmospheric implications of the title reaction have been estimated by comparing the atmospheric lifetime of ketene for the title reaction against reactions with ˙OH, H2O and NH3. On a global scale, the lifetime for the title reaction was found to be almost 70 times that for the reaction with ˙OH. However, under special conditions, where the local concentration of singlet O2 is significantly higher and/or the concentration of ˙OH is significantly lower, singlet O2 initiated oxidation could become the most significant tropospheric loss mechanism of CH2CO.
中文翻译:

单线态 (1Δg) O2 引发气相氧化,成为乙烯酮的潜在对流层衰变通道
CH2CO 被 (1Δg) O2 氧化的研究是通过高级量子化学和化学动力学计算进行的。发现该反应通过四元环状过渡态进行,这是由于在乙烯酮的 CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 键上添加了 O2 而产生的。反应能量学是使用 CCSD(T) 后校正计算的。发现过渡态的能量比分离反应物的能量低 33.6 kcal mol-1。在对流层条件下使用主方程计算的速率系数发现,在 298 K 和 1 bar 时,速率系数为 5.1 × 10-15 cm3 分子-1 s-1。通过比较标题反应与与 ̇OH、H2、O 和 NH3 反应的乙烯酮的大气寿命,估计了标题反应的大气影响。在全球范围内,研究发现标题反应的寿命几乎是与 ̇OH 反应的 70 倍。然而,在特殊条件下,单线态 O2 的局部浓度明显较高和/或 ̇OH 浓度显著降低,单线态 O2 引发的氧化可能成为 CH2CO 最显著的对流层损失机制。
键上添加了 O2 而产生的。反应能量学是使用 CCSD(T) 后校正计算的。发现过渡态的能量比分离反应物的能量低 33.6 kcal mol-1。在对流层条件下使用主方程计算的速率系数发现,在 298 K 和 1 bar 时,速率系数为 5.1 × 10-15 cm3 分子-1 s-1。通过比较标题反应与与 ̇OH、H2、O 和 NH3 反应的乙烯酮的大气寿命,估计了标题反应的大气影响。在全球范围内,研究发现标题反应的寿命几乎是与 ̇OH 反应的 70 倍。然而,在特殊条件下,单线态 O2 的局部浓度明显较高和/或 ̇OH 浓度显著降低,单线态 O2 引发的氧化可能成为 CH2CO 最显著的对流层损失机制。
更新日期:2024-12-09
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of ketene. The reaction energetics has been calculated employing post-CCSD(T) corrections. The energy of the transition state was found to be 33.6 kcal mol−1 below that of the isolated reactants. The rate coefficient, calculated using master equations under tropospheric conditions, was found to be 5.1 × 10−15 cm3 molecule−1 s−1 at 298 K and 1 bar. Atmospheric implications of the title reaction have been estimated by comparing the atmospheric lifetime of ketene for the title reaction against reactions with ˙OH, H2O and NH3. On a global scale, the lifetime for the title reaction was found to be almost 70 times that for the reaction with ˙OH. However, under special conditions, where the local concentration of singlet O2 is significantly higher and/or the concentration of ˙OH is significantly lower, singlet O2 initiated oxidation could become the most significant tropospheric loss mechanism of CH2CO.
C bond of ketene. The reaction energetics has been calculated employing post-CCSD(T) corrections. The energy of the transition state was found to be 33.6 kcal mol−1 below that of the isolated reactants. The rate coefficient, calculated using master equations under tropospheric conditions, was found to be 5.1 × 10−15 cm3 molecule−1 s−1 at 298 K and 1 bar. Atmospheric implications of the title reaction have been estimated by comparing the atmospheric lifetime of ketene for the title reaction against reactions with ˙OH, H2O and NH3. On a global scale, the lifetime for the title reaction was found to be almost 70 times that for the reaction with ˙OH. However, under special conditions, where the local concentration of singlet O2 is significantly higher and/or the concentration of ˙OH is significantly lower, singlet O2 initiated oxidation could become the most significant tropospheric loss mechanism of CH2CO.
中文翻译:

单线态 (1Δg) O2 引发气相氧化,成为乙烯酮的潜在对流层衰变通道
CH2CO 被 (1Δg) O2 氧化的研究是通过高级量子化学和化学动力学计算进行的。发现该反应通过四元环状过渡态进行,这是由于在乙烯酮的 CC






























 京公网安备 11010802027423号
京公网安备 11010802027423号