Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of absolute intramolecular distances in proteins using anomalous X-ray scattering interferometry
Nanoscale ( IF 5.8 ) Pub Date : 2024-12-09 , DOI: 10.1039/d4nr03375b Samuel Stubhan, Anna V. Baptist, Caroline Körösy, Alessandra Narducci, Gustavo Gabriel Moya Muñoz, Nicolas Wendler, Aidin Lak, Michael Sztucki, Thorben Cordes, Jan Lipfert
Nanoscale ( IF 5.8 ) Pub Date : 2024-12-09 , DOI: 10.1039/d4nr03375b Samuel Stubhan, Anna V. Baptist, Caroline Körösy, Alessandra Narducci, Gustavo Gabriel Moya Muñoz, Nicolas Wendler, Aidin Lak, Michael Sztucki, Thorben Cordes, Jan Lipfert
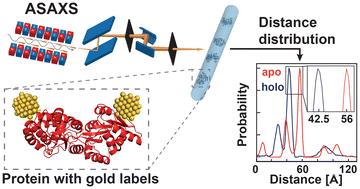
|
Biomolecular structures are typically determined using frozen or crystalline samples. Measurement of intramolecular distances in solution can provide additional insights into conformational heterogeneity and dynamics of biological macromolecules and their complexes. The established molecular ruler techniques used for this (NMR, FRET, and EPR) are, however, limited in their dynamic range and require model assumptions to determine absolute distance or distance distributions. Here, we introduce anomalous X-ray scattering interferometry (AXSI) for intramolecular distance measurements in proteins, which are labeled at two sites with small gold nanoparticles of 0.7 nm radius. We apply AXSI to two different cysteine-variants of maltose binding protein in the presence and absence of its ligand maltose and find distances in quantitative agreement with single-molecule FRET experiments. Our study shows that AXSI enables determination of intramolecular distance distributions under virtually arbitrary solution conditions and we anticipate its broad use to characterize protein conformational ensembles and dynamics.
中文翻译:

使用异常 X 射线散射干涉测量法测定蛋白质中的绝对分子内距离
生物分子结构通常使用冷冻或结晶样品来确定。测量溶液中的分子内距离可以更深入地了解生物大分子及其复合物的构象异质性和动力学。然而,用于此目的的成熟分子标尺技术(NMR、FRET 和 EPR)的动态范围有限,需要模型假设来确定绝对距离或距离分布。在这里,我们介绍了用于蛋白质分子内距离测量的异常 X 射线散射干涉测量法 (AXSI),这些蛋白质在两个位点用半径为 0.7 nm 的小金纳米颗粒标记。在麦芽糖配体麦芽糖存在和不存在的情况下,我们将 AXSI 应用于麦芽糖结合蛋白的两种不同的半胱氨酸变体,并找到与单分子 FRET 实验定量一致的距离。我们的研究表明,AXSI 能够在几乎任意的溶液条件下确定分子内距离分布,我们预计它将广泛用于表征蛋白质构象集合和动力学。
更新日期:2024-12-09
中文翻译:

使用异常 X 射线散射干涉测量法测定蛋白质中的绝对分子内距离
生物分子结构通常使用冷冻或结晶样品来确定。测量溶液中的分子内距离可以更深入地了解生物大分子及其复合物的构象异质性和动力学。然而,用于此目的的成熟分子标尺技术(NMR、FRET 和 EPR)的动态范围有限,需要模型假设来确定绝对距离或距离分布。在这里,我们介绍了用于蛋白质分子内距离测量的异常 X 射线散射干涉测量法 (AXSI),这些蛋白质在两个位点用半径为 0.7 nm 的小金纳米颗粒标记。在麦芽糖配体麦芽糖存在和不存在的情况下,我们将 AXSI 应用于麦芽糖结合蛋白的两种不同的半胱氨酸变体,并找到与单分子 FRET 实验定量一致的距离。我们的研究表明,AXSI 能够在几乎任意的溶液条件下确定分子内距离分布,我们预计它将广泛用于表征蛋白质构象集合和动力学。






























 京公网安备 11010802027423号
京公网安备 11010802027423号