当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a New Vaccine Adjuvant System Based on the Combination of the Synthetic TLR4 Agonist FP20 and a Synthetic QS-21 Variant
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-12-08 , DOI: 10.1021/acs.jmedchem.4c02392 Mohammed Monsoor Shaik, Samuel Pasco, Alessio Romerio, Carlo Pifferi, Silvia Sesana, Francesca Re, Charl Xavier Bezuidenhout, Silvia Bracco, Alberto Fernandez-Tejada, Juan Anguita, Francesco Peri
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-12-08 , DOI: 10.1021/acs.jmedchem.4c02392 Mohammed Monsoor Shaik, Samuel Pasco, Alessio Romerio, Carlo Pifferi, Silvia Sesana, Francesca Re, Charl Xavier Bezuidenhout, Silvia Bracco, Alberto Fernandez-Tejada, Juan Anguita, Francesco Peri
|
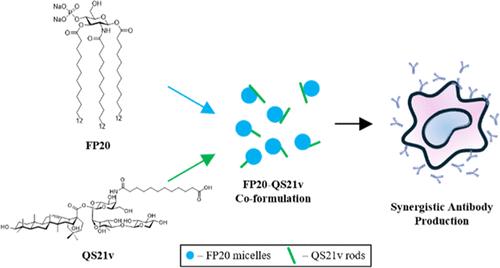
In this study, we formulated an alternative to AS01b by combining FP20, a synthetic TLR4 agonist, and QS21v, a minimal saponin adjuvant, aiming to improve the vaccine efficacy and stability. The phase transition temperature of FP20 was determined by using differential scanning calorimetry to be 43.9 °C, providing a foundation for the formulation process. The coformulation was prepared using a dry film method for even adjuvant distribution. Characterization by dynamic light scattering and nanoparticle tracking analysis revealed a uniform particle size distribution of ∼120 nm. Cryogenic electron microscopy (CryoEM) revealed nanosized interactions between FP20 and QS21v, forming stable structures that likely enhanced the antigen presentation and immune activation. These physicochemical properties contributed to a robust in vivo synergy, where the coformulation elicited significantly higher antigen-specific antibody titers compared to individual adjuvants. These findings suggest that the FP20+QS21v coformulation provides a potent, stable, and safer alternative to traditional adjuvants, enhancing both vaccine efficacy and immunogenicity.
中文翻译:

基于合成 TLR4 激动剂 FP20 和合成 QS-21 变体组合的新型疫苗佐剂系统的开发
在这项研究中,我们通过将合成 TLR4 激动剂 FP20 和最小皂苷佐剂 QS21v 相结合,制定了 AS01b 的替代品,旨在提高疫苗的有效性和稳定性。使用差示扫描量热法确定 FP20 的相变温度为 43.9 °C,为配方过程提供了基础。使用干膜法制备共制剂,以实现佐剂均匀分布。通过动态光散射和纳米颗粒跟踪分析进行表征,发现颗粒粒度分布均匀,为 ∼120 nm。低温电子显微镜 (CryoEM) 揭示了 FP20 和 QS21v 之间的纳米级相互作用,形成了稳定的结构,可能增强了抗原呈递和免疫激活。这些物理化学特性有助于强大的体内协同作用,与单个佐剂相比,共制剂引起的抗原特异性抗体滴度明显更高。这些发现表明,FP20+QS21v 联合制剂为传统佐剂提供了一种有效、稳定且更安全的替代品,提高了疫苗功效和免疫原性。
更新日期:2024-12-08
中文翻译:

基于合成 TLR4 激动剂 FP20 和合成 QS-21 变体组合的新型疫苗佐剂系统的开发
在这项研究中,我们通过将合成 TLR4 激动剂 FP20 和最小皂苷佐剂 QS21v 相结合,制定了 AS01b 的替代品,旨在提高疫苗的有效性和稳定性。使用差示扫描量热法确定 FP20 的相变温度为 43.9 °C,为配方过程提供了基础。使用干膜法制备共制剂,以实现佐剂均匀分布。通过动态光散射和纳米颗粒跟踪分析进行表征,发现颗粒粒度分布均匀,为 ∼120 nm。低温电子显微镜 (CryoEM) 揭示了 FP20 和 QS21v 之间的纳米级相互作用,形成了稳定的结构,可能增强了抗原呈递和免疫激活。这些物理化学特性有助于强大的体内协同作用,与单个佐剂相比,共制剂引起的抗原特异性抗体滴度明显更高。这些发现表明,FP20+QS21v 联合制剂为传统佐剂提供了一种有效、稳定且更安全的替代品,提高了疫苗功效和免疫原性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号