当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of novel diaryl-substituted fused nitrogen heterocycles as tubulin polymerization inhibitors to overcome multidrug resistance in vitro and in vivo
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-08 , DOI: 10.1016/j.ejmech.2024.117130
Fuhao Jiang, Min Yu, Yang Wang
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-08 , DOI: 10.1016/j.ejmech.2024.117130
Fuhao Jiang, Min Yu, Yang Wang
|
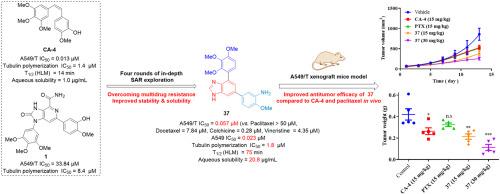
Microtubule-targeting agents (MTAs) are considered as one of the most successful chemotherapy drugs for lung adenocarcinoma (LUAD). However, the clinical application of MTAs is often significantly plagued by multidrug resistance (MDR). To overcome this limitation in the quest of more effective MTAs for tumor therapy, a series of novel diaryl-substituted nitrogenous fused heterocycles were designed, synthesized and evaluated. Through four rounds of structure-activity relationship studies, the benzoimidazole derivative 37 was identified as a potent cytotoxic agent against both paclitaxel-sensitive and -resistant A549 (A549/T) cells, effectively overcoming multidrug resistance of A549/T cells against various MTAs. Mechanistic investigations revealed that 37 could disrupt microtubule assembly and induce cell cycle arrest at the G2/M phase, and hence trigger the cell apoptosis. Furthermore, 37 was found to be a poor substrate for P -glycoprotein (P -gp), a major contributor to multidrug resistance, and could reduce the level of P -gp in resistant cells, thereby effectively overcoming P -gp-mediated multidrug resistance. Notably, 37 exhibited higher liver microsomal stability and better water solubility than those of the reference combretastatin A-4 (CA-4). In vivo studies using an A549/T xenograft model demonstrated that 37 significantly inhibited tumor growth without obvious toxicity, outperforming the positive controls CA-4 and paclitaxel. As a novel tubulin polymerization inhibitor, compound 37 is marked by potent anticancer activity and remarkable anti -MDR properties. These salient features, coupled with the low toxicity of 37 , would render it quite promising as a lead for further drug development towards clinical treatment of multidrug-resistant LUAD.
中文翻译:

新型二芳基取代的熔融氮杂环作为微管蛋白聚合抑制剂的设计、合成和生物学评价,以克服体内外多药耐药性
微管靶向剂 (MTA) 被认为是肺腺癌 (LUAD) 最成功的化疗药物之一。然而,MTA 的临床应用往往受到多药耐药 (MDR) 的严重困扰。为了克服这一限制,寻求更有效的 MTA 用于肿瘤治疗,设计、合成和评估了一系列新型二芳基取代的含氮稠合杂环。通过四轮构效关系研究,确定苯并咪唑衍生物 37 是一种有效的细胞毒剂,可对抗紫杉醇敏感和耐药的 A549 (A549/T) 细胞,有效克服 A549/T 细胞对各种 MTA 的多药耐药性。机制调查显示,37 可以破坏微管组装并诱导细胞周期停滞在 G2/M 期,从而触发细胞凋亡。此外,发现 37 是 P-糖蛋白 (P-gp) 的不良底物,P-糖蛋白是导致多药耐药的主要因素,可以降低耐药细胞中 P-gp 的水平,从而有效克服 P-gp 介导的多药耐药。值得注意的是,37 个表现出比参考 combretastatin A-4 (CA-4) 更高的肝脏微粒体稳定性和更好的水溶性。使用 A549/T 异种移植模型的体内研究表明,37 显着抑制肿瘤生长,无明显毒性,优于阳性对照 CA-4 和紫杉醇。作为一种新型微管蛋白聚合抑制剂,化合物 37 具有强大的抗癌活性和显著的抗 MDR 特性。这些突出的特点,再加上 37 的低毒性,将使其非常有希望成为进一步药物开发以临床治疗多重耐药 LUAD 的先导药物。
更新日期:2024-12-08
中文翻译:

新型二芳基取代的熔融氮杂环作为微管蛋白聚合抑制剂的设计、合成和生物学评价,以克服体内外多药耐药性
微管靶向剂 (MTA) 被认为是肺腺癌 (LUAD) 最成功的化疗药物之一。然而,MTA 的临床应用往往受到多药耐药 (MDR) 的严重困扰。为了克服这一限制,寻求更有效的 MTA 用于肿瘤治疗,设计、合成和评估了一系列新型二芳基取代的含氮稠合杂环。通过四轮构效关系研究,确定苯并咪唑衍生物 37 是一种有效的细胞毒剂,可对抗紫杉醇敏感和耐药的 A549 (A549/T) 细胞,有效克服 A549/T 细胞对各种 MTA 的多药耐药性。机制调查显示,37 可以破坏微管组装并诱导细胞周期停滞在 G2/M 期,从而触发细胞凋亡。此外,发现 37 是 P-糖蛋白 (P-gp) 的不良底物,P-糖蛋白是导致多药耐药的主要因素,可以降低耐药细胞中 P-gp 的水平,从而有效克服 P-gp 介导的多药耐药。值得注意的是,37 个表现出比参考 combretastatin A-4 (CA-4) 更高的肝脏微粒体稳定性和更好的水溶性。使用 A549/T 异种移植模型的体内研究表明,37 显着抑制肿瘤生长,无明显毒性,优于阳性对照 CA-4 和紫杉醇。作为一种新型微管蛋白聚合抑制剂,化合物 37 具有强大的抗癌活性和显著的抗 MDR 特性。这些突出的特点,再加上 37 的低毒性,将使其非常有希望成为进一步药物开发以临床治疗多重耐药 LUAD 的先导药物。































 京公网安备 11010802027423号
京公网安备 11010802027423号