当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Diaryl Ketone Synthesis by N–C Cleavage: Direct Negishi Cross-Coupling of Primary Amides by Site-Selective N,N-Di-Boc Activation
Organic Letters ( IF 4.9 ) Pub Date : 2016-11-08 00:00:00 , DOI: 10.1021/acs.orglett.6b02952 Shicheng Shi 1 , Michal Szostak 1
Organic Letters ( IF 4.9 ) Pub Date : 2016-11-08 00:00:00 , DOI: 10.1021/acs.orglett.6b02952 Shicheng Shi 1 , Michal Szostak 1
Affiliation
|
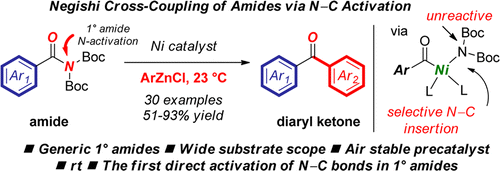
A general Negishi acylation of primary amides enabled by a combination of site-selective N,N-di-Boc activation and nickel catalysis is reported for the first time. The reaction is promoted by a bench-stable, inexpensive Ni catalyst. The reaction shows excellent functional group compatibility, affording functionalized diaryl ketones by selective N–C cleavage. Most notably, this protocol represents the first amide cross-coupling by direct metal insertion of simple and readily available primary amides. The overall strategy by N,N-di-Boc activation/metal insertion is suitable for a broad range of coupling protocols via acylmetals. Mechanistic experiments suggest high reactivity of N,N-di-Boc activated 1° amides in direct amide C–N cross-couplings.
中文翻译:

N-C裂解合成镍催化的二芳基酮:通过位点选择性N,N -Di-Boc活化直接将伯酰胺进行Negishi交叉偶联
首次报道了通过位点选择性N,N -di-Boc活化和镍催化的组合实现的伯酰胺的一般Negishi酰化反应。稳定的,廉价的镍催化剂促进了反应的进行。该反应显示出优异的官能团相容性,可通过选择性N-C裂解获得官能化的二芳基酮。最值得注意的是,该方案代表了通过直接金属插入简单易得的伯酰胺而进行的第一个酰胺交叉偶联。N,N -di-Boc活化/金属插入的整体策略适用于广泛的通过酰基金属的偶联方案。力学实验表明N,N具有高反应活性-di-Boc在酰胺直接C–N交叉偶联中活化了1°酰胺。
更新日期:2016-11-08
中文翻译:

N-C裂解合成镍催化的二芳基酮:通过位点选择性N,N -Di-Boc活化直接将伯酰胺进行Negishi交叉偶联
首次报道了通过位点选择性N,N -di-Boc活化和镍催化的组合实现的伯酰胺的一般Negishi酰化反应。稳定的,廉价的镍催化剂促进了反应的进行。该反应显示出优异的官能团相容性,可通过选择性N-C裂解获得官能化的二芳基酮。最值得注意的是,该方案代表了通过直接金属插入简单易得的伯酰胺而进行的第一个酰胺交叉偶联。N,N -di-Boc活化/金属插入的整体策略适用于广泛的通过酰基金属的偶联方案。力学实验表明N,N具有高反应活性-di-Boc在酰胺直接C–N交叉偶联中活化了1°酰胺。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号