当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A New platform of Electrowetting-on-dielectric Digital Microfluidics for Rapid detection of Early-stage Hepatocellular Carcinoma(HCC) specific Biomarker
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2024-12-07 , DOI: 10.1016/j.aca.2024.343533 Wenjie Zhu, Hong Qian, Yasheng Cao, Wei Xia, Xilong Wang, Jing Jin, Xin Wang, Hao Zhang, Dongsheng Liu, Ying Chen
中文翻译:

用于快速检测早期肝细胞癌 (HCC) 特异性生物标志物的电润湿数字微流控新平台
肝细胞癌 (HCC) 的早期检测对于提高患者生存率至关重要。HCC 的早期诊断可以显着提高治疗结果并减缓疾病进展。肿瘤标志物抗原检测是 HCC 的重要诊断方法之一。然而,传统的抗原检测方法通常依赖于重型检测设备,周转时间长,并且必须在实验室环境中进行。因此,显然需要一种便携式、低技能、快速的样本到结果检测方法来检测早期 HCC 生物标志物。
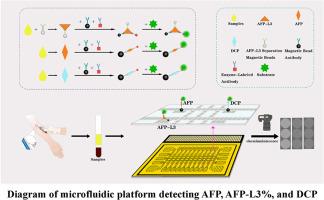
我们提出了一种基于电湿介电数字微流控 (EWOD-DMF) 的新平台,用于检测早期 HCC 生物标志物,能够定量测量甲胎蛋白 (AFP)、AFP-L3 在总 AFP 中的比例 (AFP-L3%) 和 Des-Gamma-Carboxy 凝血酶原 (DCP)。首先,通过微流控系统处理血清样品,在 10 分钟内实现 AFP-L3 的分离。接下来,在 15 分钟内进行免疫测定,使用磁性颗粒捕获生物标志物,如 AFP、AFP-L3 和 DCP,然后进行产生可检测信号的酶促反应。每个芯片可以同时检测 5 个不同样品中的 3 个生物标志物,总共可以检测 15 个靶标,每个生物标志物检测只需要大约 2.4 μL 的血清。最终,使用专用软件分析数据以定量测量 HCC 生物标志物。AFP 或 AFP-L3 和 DCP 的检测限分别为 0.24 ng/mL 和 1.89 ng/mL。
本研究提出了一种用于早期 HCC 诊断的 EWOD-DMF 平台,能够同时检测多个样本和生物标志物,从而提高检测效率和诊断准确性。此外,该平台具有 POCT 功能,具有便携性和成本效益方面的优势,为临床医生和基层医疗机构提供快速便捷的早期 HCC 诊断解决方案。
更新日期:2024-12-07
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2024-12-07 , DOI: 10.1016/j.aca.2024.343533 Wenjie Zhu, Hong Qian, Yasheng Cao, Wei Xia, Xilong Wang, Jing Jin, Xin Wang, Hao Zhang, Dongsheng Liu, Ying Chen
|
Background
The early detection of Hepatocellular Carcinoma (HCC) is crucial for improving patient survival rates.Early diagnosis of HCC can significantly enhance treatment outcomes and reduce disease progression. Antigen detection of tumor markers is one of the important diagnostic methods for HCC. However, Traditional antigen detection methods often rely on heavy detection equipment, involve lengthy turnaround times, and must be conducted in laboratory settings. Therefore, there is a clear need for a portable, low-skill, rapid sample-to-result detection method for early HCC biomarkers.Results
We propose a new platform based on electrowetting-on-dielectric digital microfluidic(EWOD-DMF) for the detection of early-stage HCC biomarkers, enabling the quantitative measurement of Alpha-Fetoprotein (AFP), the proportion of AFP-L3 in total AFP (AFP-L3%), and Des-Gamma-Carboxy Prothrombin (DCP). First, serum samples are processed through the microfluidic system, achieving the separation of AFP-L3 within 10 minutes. Next, immunoassays are performed within 15 minutes, using magnetic particles to capture biomarkers such as AFP, AFP-L3, and DCP, followed by enzymatic reactions that generate detectable signals. Each chip can simultaneously detect three biomarkers from five different samples, allowing for a total of fifteen targets to be tested, with only approximately 2.4 μL of serum required for each biomarker detection. Ultimately, data are analyzed with dedicated software to quantitatively measure the HCC biomarkers. The detection limits for AFP or AFP-L3 and for DCP are 0.24 ng/mL and 1.89 ng/mL, respectively.Significance
This study presents a EWOD-DMF platform for early-stage HCC diagnosis, capable of simultaneously detecting multiple samples and biomarkers, thus improving detection efficiency and diagnostic accuracy. Moreover, the platform has POCT capability, with advantages in portability and cost-effectiveness, providing clinicians and primary healthcare institutions with a fast and convenient solution for early-stage HCC diagnosis.中文翻译:

用于快速检测早期肝细胞癌 (HCC) 特异性生物标志物的电润湿数字微流控新平台
背景
肝细胞癌 (HCC) 的早期检测对于提高患者生存率至关重要。HCC 的早期诊断可以显着提高治疗结果并减缓疾病进展。肿瘤标志物抗原检测是 HCC 的重要诊断方法之一。然而,传统的抗原检测方法通常依赖于重型检测设备,周转时间长,并且必须在实验室环境中进行。因此,显然需要一种便携式、低技能、快速的样本到结果检测方法来检测早期 HCC 生物标志物。
结果
我们提出了一种基于电湿介电数字微流控 (EWOD-DMF) 的新平台,用于检测早期 HCC 生物标志物,能够定量测量甲胎蛋白 (AFP)、AFP-L3 在总 AFP 中的比例 (AFP-L3%) 和 Des-Gamma-Carboxy 凝血酶原 (DCP)。首先,通过微流控系统处理血清样品,在 10 分钟内实现 AFP-L3 的分离。接下来,在 15 分钟内进行免疫测定,使用磁性颗粒捕获生物标志物,如 AFP、AFP-L3 和 DCP,然后进行产生可检测信号的酶促反应。每个芯片可以同时检测 5 个不同样品中的 3 个生物标志物,总共可以检测 15 个靶标,每个生物标志物检测只需要大约 2.4 μL 的血清。最终,使用专用软件分析数据以定量测量 HCC 生物标志物。AFP 或 AFP-L3 和 DCP 的检测限分别为 0.24 ng/mL 和 1.89 ng/mL。
意义
本研究提出了一种用于早期 HCC 诊断的 EWOD-DMF 平台,能够同时检测多个样本和生物标志物,从而提高检测效率和诊断准确性。此外,该平台具有 POCT 功能,具有便携性和成本效益方面的优势,为临床医生和基层医疗机构提供快速便捷的早期 HCC 诊断解决方案。






























 京公网安备 11010802027423号
京公网安备 11010802027423号