当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypoxia-selective prodrug restrains tumor cells through triggering mitophagy and inducing apoptosis
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-07 , DOI: 10.1016/j.ejmech.2024.117155 Fangjie Wang, Lairong Song, Qianqian Xu, Ang Jia, Xiangwei Meng, Hongfei Jiang, Renshuai Zhang
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-07 , DOI: 10.1016/j.ejmech.2024.117155 Fangjie Wang, Lairong Song, Qianqian Xu, Ang Jia, Xiangwei Meng, Hongfei Jiang, Renshuai Zhang
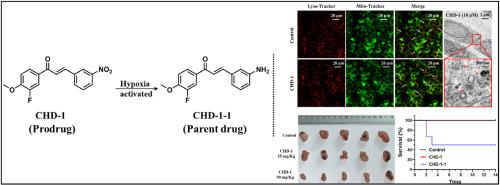
|
Hypoxia is a common feature of various solid tumors, which reduces the sensitivity of tumor cells to both radiotherapy and chemotherapy. However, hypoxia also presents an opportunity for tumor-selective therapy. The prodrug strategy, leveraging the hypoxic nature of the tumor microenvironment, shows significant potential for clinical application. Here we present CHD-1 , a hypoxia-activated antitumor prodrug that activates in hypoxic environments, effectively inhibiting hypoxic tumor cells while exhibiting no toxicity to normoxic cells. CHD-1 impairs mitochondrial morphology and membrane potential of hypoxic tumor cells, further triggers excessive mitophagy and induces apoptosis. Moreover, prodrug CHD-1 significantly inhibits HeLa xenograft growth in vivo , and shows lower toxicity than parent molecule in an acute toxicity assessment in animal models. This study introduces a promising hypoxia-activated antitumor prodrug with strong potential for further development in hypoxic tumor therapy.
中文翻译:

缺氧选择性前药通过触发线粒体自噬和诱导细胞凋亡来抑制肿瘤细胞
缺氧是各种实体瘤的共同特征,它降低了肿瘤细胞对放疗和化疗的敏感性。然而,缺氧也为肿瘤选择性治疗提供了机会。前药策略利用肿瘤微环境的缺氧性质,显示出巨大的临床应用潜力。在这里,我们介绍了 CHD-1,一种缺氧激活的抗肿瘤前药,在缺氧环境中激活,有效抑制缺氧肿瘤细胞,同时对常氧细胞无毒性。CHD-1 损害缺氧肿瘤细胞的线粒体形态和膜电位,进一步触发过度线粒体自噬并诱导细胞凋亡。此外,前药 CHD-1 在体内显着抑制 HeLa 异种移植物的生长,并且在动物模型的急性毒性评估中显示出比母体分子更低的毒性。本研究介绍了一种有前途的缺氧激活抗肿瘤前药,在缺氧肿瘤治疗中具有很大的进一步发展潜力。
更新日期:2024-12-07
中文翻译:

缺氧选择性前药通过触发线粒体自噬和诱导细胞凋亡来抑制肿瘤细胞
缺氧是各种实体瘤的共同特征,它降低了肿瘤细胞对放疗和化疗的敏感性。然而,缺氧也为肿瘤选择性治疗提供了机会。前药策略利用肿瘤微环境的缺氧性质,显示出巨大的临床应用潜力。在这里,我们介绍了 CHD-1,一种缺氧激活的抗肿瘤前药,在缺氧环境中激活,有效抑制缺氧肿瘤细胞,同时对常氧细胞无毒性。CHD-1 损害缺氧肿瘤细胞的线粒体形态和膜电位,进一步触发过度线粒体自噬并诱导细胞凋亡。此外,前药 CHD-1 在体内显着抑制 HeLa 异种移植物的生长,并且在动物模型的急性毒性评估中显示出比母体分子更低的毒性。本研究介绍了一种有前途的缺氧激活抗肿瘤前药,在缺氧肿瘤治疗中具有很大的进一步发展潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号