当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and antitumor evaluation of quinazoline-4-tetrahydroquinoline chemotypes as novel tubulin polymerization inhibitors targeting the colchicine site
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-06 , DOI: 10.1016/j.ejmech.2024.117139 Qinhuai Lai, Zhijia Wang, Chengyong Wu, Ruofei Zhang, Leyan Li, Yiran Tao, Dan Mo, Jifa Zhang, Lantu Gou, Yuxi Wang
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-06 , DOI: 10.1016/j.ejmech.2024.117139 Qinhuai Lai, Zhijia Wang, Chengyong Wu, Ruofei Zhang, Leyan Li, Yiran Tao, Dan Mo, Jifa Zhang, Lantu Gou, Yuxi Wang
|
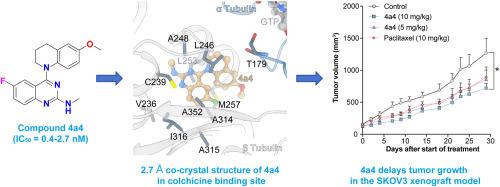
We designed, synthesized, and evaluated the antitumor activity of a series of novel quinazoline-4-(6-methoxytetrahydroquinoline) analogues. Among the tested compounds, 4a4 exhibited the most potent antiproliferative activities across four human cancer cell lines with half-maximal inhibitory concentration (IC50 ) values ranging from 0.4 to 2.7 nM, more potent than the lead compound. The 2.71 Å resolution co-crystal structure of 4a4 with tubulin (PDB code: 8YER) confirmed its critical binding at the colchicine site. Moreover, 4a4 inhibited the polymerization of tubulin, colony formation, and tumor cell migration, while inducing G2/M phase arrest and apoptosis. In vivo , 4a4 significantly delayed primary tumor growth in the SKOV3 xenograft model without obvious side effect. Our research enhances the structure-activity relationships (SARs) understanding of the quinazoline-4-tetrahydroquinoline scaffold and provides new insights for potential structural optimization and the development of novel colchicine binding site inhibitors (CBSIs).
中文翻译:

喹唑啉-4-四氢喹啉化学型作为靶向秋水仙碱位点的新型微管蛋白聚合抑制剂的设计、合成和抗肿瘤评价
我们设计、合成和评价了一系列新型喹唑啉-4-(6-甲氧基四氢喹啉)类似物的抗肿瘤活性。在测试的化合物中,4a4 在四种人癌细胞系中表现出最有效的抗增殖活性,半数最大抑制浓度 (IC50) 值范围为 0.4 至 2.7 nM,比先导化合物更有效。4a4 与微管蛋白(PDB 代码:8YER)的 2.71 Å 分辨率共晶体结构证实了它在秋水仙碱位点的关键结合。此外,4a4 抑制微管蛋白的聚合、集落形成和肿瘤细胞迁移,同时诱导 G2/M 期停滞和细胞凋亡。在体内,4a4 在 SKOV3 异种移植模型中显着延迟了原发性肿瘤的生长,没有明显的副作用。我们的研究增强了对喹唑啉-4-四氢喹啉支架的构效关系 (SARs) 的理解,并为潜在的结构优化和新型秋水仙碱结合位点抑制剂 (CBSIs) 的开发提供了新的见解。
更新日期:2024-12-06
中文翻译:

喹唑啉-4-四氢喹啉化学型作为靶向秋水仙碱位点的新型微管蛋白聚合抑制剂的设计、合成和抗肿瘤评价
我们设计、合成和评价了一系列新型喹唑啉-4-(6-甲氧基四氢喹啉)类似物的抗肿瘤活性。在测试的化合物中,4a4 在四种人癌细胞系中表现出最有效的抗增殖活性,半数最大抑制浓度 (IC50) 值范围为 0.4 至 2.7 nM,比先导化合物更有效。4a4 与微管蛋白(PDB 代码:8YER)的 2.71 Å 分辨率共晶体结构证实了它在秋水仙碱位点的关键结合。此外,4a4 抑制微管蛋白的聚合、集落形成和肿瘤细胞迁移,同时诱导 G2/M 期停滞和细胞凋亡。在体内,4a4 在 SKOV3 异种移植模型中显着延迟了原发性肿瘤的生长,没有明显的副作用。我们的研究增强了对喹唑啉-4-四氢喹啉支架的构效关系 (SARs) 的理解,并为潜在的结构优化和新型秋水仙碱结合位点抑制剂 (CBSIs) 的开发提供了新的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号