当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconstituting the immune killing functions and improving the pharmacokinetics of nanobodies by rhamnolipid conjugation
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-12-09 , DOI: 10.1016/j.jconrel.2024.11.080 Yanchun Li, Dan Li, Han Lin, Di Wang, Jie Zhao, Zheng Wang, Haofei Hong, Zhimeng Wu
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-12-09 , DOI: 10.1016/j.jconrel.2024.11.080 Yanchun Li, Dan Li, Han Lin, Di Wang, Jie Zhao, Zheng Wang, Haofei Hong, Zhimeng Wu
|
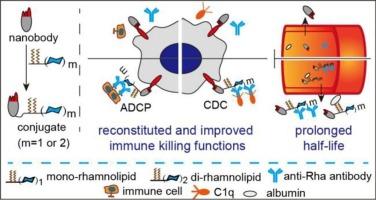
Nanobodies (Nbs) hold great promise as next-generation cancer immunotherapies, but their efficacy is hindered by their poor pharmacokinetics and the inability to trigger Fc-mediated immune killing functions. To address these limitations, we designed and synthesized rhamnolipid-modified Nbs as a type of antibody-recruiting molecule by site-specifically conjugating EGFR-targeting Nb 7D12 to a series of rhamnolipid derivatives, and their biological profiles were evaluated in vitro and in vivo . Investigation of the structure-activity relationship revealed that the number of rhamnose (Rha) units and the length of the PEG linker in the conjugates affected anti-tumor activities. Conjugate R5 , which contained two Rha units and a PEG2 linker, exhibited the most potent antibody-dependent cell-mediated phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC) activities. In vivo , R5 had a significantly longer half-life because of its ability to bind to serum albumin and endogenous anti-Rha antibodies, and it demonstrated potent in vivo antitumor activity in a xenograft mouse model of A431 tumor. Our findings highlight the potential of rhamnolipidation as a strategy to enhance the efficacy of Nbs in cancer immunotherapy and provide a cost-effective platform for improving the therapeutic efficiency of Nbs.
中文翻译:

通过鼠李糖脂偶联重构纳米抗体的免疫杀伤功能并改善其药代动力学
纳米抗体 (Nbs) 作为下一代癌症免疫疗法具有很大的前景,但其疗效受到其药代动力学差和无法触发 Fc 介导的免疫杀伤功能的阻碍。为了解决这些限制,我们通过将靶向 EGFR 的 Nb 7D12 与一系列鼠李糖脂衍生物定点特异性偶联,设计并合成了鼠李糖脂修饰的 Nbs 作为一种抗体募集分子,并在体外和体内评估了它们的生物学特征。结构-活性关系的研究表明,偶联物中鼠李糖 (Rha) 单位的数量和 PEG 接头的长度会影响抗肿瘤活性。偶联物 R5 包含两个 Rha 单元和一个 PEG2 接头,表现出最有效的抗体依赖性细胞介导的吞噬作用 (ADCP) 和补体依赖性细胞毒性 (CDC) 活性。在体内,R5 具有显著更长的半衰期,因为它能够与血清白蛋白和内源性抗 Rha 抗体结合,并且在 A431 肿瘤的异种移植小鼠模型中显示出强大的体内抗肿瘤活性。我们的研究结果强调了鼠李糖脂作为一种策略的潜力,可以提高 Nbs 在癌症免疫治疗中的疗效,并为提高 Nbs 的治疗效率提供具有成本效益的平台。
更新日期:2024-12-09
中文翻译:

通过鼠李糖脂偶联重构纳米抗体的免疫杀伤功能并改善其药代动力学
纳米抗体 (Nbs) 作为下一代癌症免疫疗法具有很大的前景,但其疗效受到其药代动力学差和无法触发 Fc 介导的免疫杀伤功能的阻碍。为了解决这些限制,我们通过将靶向 EGFR 的 Nb 7D12 与一系列鼠李糖脂衍生物定点特异性偶联,设计并合成了鼠李糖脂修饰的 Nbs 作为一种抗体募集分子,并在体外和体内评估了它们的生物学特征。结构-活性关系的研究表明,偶联物中鼠李糖 (Rha) 单位的数量和 PEG 接头的长度会影响抗肿瘤活性。偶联物 R5 包含两个 Rha 单元和一个 PEG2 接头,表现出最有效的抗体依赖性细胞介导的吞噬作用 (ADCP) 和补体依赖性细胞毒性 (CDC) 活性。在体内,R5 具有显著更长的半衰期,因为它能够与血清白蛋白和内源性抗 Rha 抗体结合,并且在 A431 肿瘤的异种移植小鼠模型中显示出强大的体内抗肿瘤活性。我们的研究结果强调了鼠李糖脂作为一种策略的潜力,可以提高 Nbs 在癌症免疫治疗中的疗效,并为提高 Nbs 的治疗效率提供具有成本效益的平台。






























 京公网安备 11010802027423号
京公网安备 11010802027423号