当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis and in Vitro Anti-Tumor-Promoting Activities of Racemic Acetophenone Monomers from Acronychia trifoliolata
Journal of Natural Products ( IF 3.3 ) Pub Date : 2016-11-07 00:00:00 , DOI: 10.1021/acs.jnatprod.6b00646 Chihiro Morita,Yukiko Kobayashi,Yohei Saito,Katsunori Miyake,Harukuni Tokuda,Nobutaka Suzuki,Eiichiro Ichiishi,Kuo-Hsiung Lee,Kyoko Nakagawa-Goto
Journal of Natural Products ( IF 3.3 ) Pub Date : 2016-11-07 00:00:00 , DOI: 10.1021/acs.jnatprod.6b00646 Chihiro Morita,Yukiko Kobayashi,Yohei Saito,Katsunori Miyake,Harukuni Tokuda,Nobutaka Suzuki,Eiichiro Ichiishi,Kuo-Hsiung Lee,Kyoko Nakagawa-Goto
|
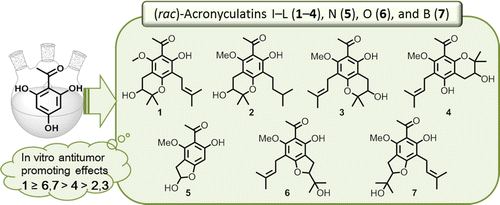
Six acetophenone derivatives, acronyculatins I (1), J (2), K (3), L (4), N (5), and O (6), were recently isolated from Acronychia trifoliolata, and the structure of the known acronyculatin B (7) was revised. Because of the limited quantities of isolated products as well as their structure similarity, racemic acronyculatins I–L, N, O, and B (1–7) were synthesized to confirm their structures and to obtain sufficient material for biological evaluation. Trihydroxyacetophenone was converted to the target compounds by various sequences of hydroxy group protection, allylation or prenylation, and epoxidation followed by cyclization. C-Prenylations were carried out by direct addition of a prenyl group or through 1,3- or 3,3-sigmatropic rearrangement. The synthesized racemic compounds were evaluated in an anti-tumor-promoting assay using the Epstein–Barr virus early antigen (EBV-EA) activation induced by 12-O-tetradecanoylphorbol-13-acetate in Raji cells. All tested compounds significantly inhibited EBV-EA activation. Especially, racemic acronyculatin I (1) displayed the most potent inhibitory effects, with an IC50 value of 7.3 μM.
中文翻译:

三叶草冠外消旋苯乙酮单体的全合成及体外抗肿瘤活性
六苯乙酮衍生物,acronyculatins我(1),J(2),K(3),L(4),N(5)和O(6),最近从Acronychia trifoliolata,和已知的acronyculatin的结构中分离出来。B(7)被修改。由于分离产品的数量有限,以及它们的结构相似性,所以外消旋的肩甲素I–L,N,O和B(1 – 7合成)以确认其结构并获得足够的材料用于生物学评估。通过羟基保护,烯丙基化或烯丙基化,环氧化和环化的各种顺序,将三羟基苯乙酮转化为目标化合物。Ç -Prenylations通过直接加入异戊烯基或通过1,3-或3,3-σ重排进行。通过在Raji细胞中由12 - O-十四烷酰phorbol-13-乙酸酯诱导的爱泼斯坦-巴尔病毒早期抗原(EBV-EA)活化,在抗肿瘤促进试验中评估了合成的外消旋化合物。所有测试的化合物均显着抑制EBV-EA活化。尤其是外消旋顶体素I(1)显示出最有效的抑制作用,IC 50值为7.3μM。
更新日期:2016-11-07
中文翻译:

三叶草冠外消旋苯乙酮单体的全合成及体外抗肿瘤活性
六苯乙酮衍生物,acronyculatins我(1),J(2),K(3),L(4),N(5)和O(6),最近从Acronychia trifoliolata,和已知的acronyculatin的结构中分离出来。B(7)被修改。由于分离产品的数量有限,以及它们的结构相似性,所以外消旋的肩甲素I–L,N,O和B(1 – 7合成)以确认其结构并获得足够的材料用于生物学评估。通过羟基保护,烯丙基化或烯丙基化,环氧化和环化的各种顺序,将三羟基苯乙酮转化为目标化合物。Ç -Prenylations通过直接加入异戊烯基或通过1,3-或3,3-σ重排进行。通过在Raji细胞中由12 - O-十四烷酰phorbol-13-乙酸酯诱导的爱泼斯坦-巴尔病毒早期抗原(EBV-EA)活化,在抗肿瘤促进试验中评估了合成的外消旋化合物。所有测试的化合物均显着抑制EBV-EA活化。尤其是外消旋顶体素I(1)显示出最有效的抑制作用,IC 50值为7.3μM。































 京公网安备 11010802027423号
京公网安备 11010802027423号