当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-principles study of the hydrogen storage properties of Irida-graphene
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-06 , DOI: 10.1039/d4cp03381g Yanhong Sun, Yuhong Chen, Menglin Yang, Kun Zhou, Jialin Sun, Kongyang Zhao, Lai Xu
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-06 , DOI: 10.1039/d4cp03381g Yanhong Sun, Yuhong Chen, Menglin Yang, Kun Zhou, Jialin Sun, Kongyang Zhao, Lai Xu
|
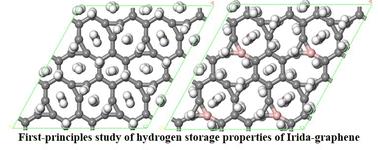
The hydrogen storage properties of the 2D carbon allotrope Irida-graphene (IG) were investigated using first-principles calculation. The intrinsic IG adsorption energy for H2 is only −0.06 eV, significantly lower than the effective adsorption threshold. To improve its hydrogen storage capabilities, IG was doped with boron (B) and modified with sodium (Na). It was found that both 2Na@IG and 2Na@BIG systems could adsorb 8 pairs of H2. However, the average adsorption energy of H2 in the 2Na@BIG system (−0.145 eV) is higher compared to that in the 2Na@IG system (−0.134 eV), and the adsorption capacity (14.6 wt%) was superior to that of the 2Na@IG system (14.5 wt%). The introduction of B created an electron-deficient structure (BIG), enhancing electron transfer between Na and the substrate to improve Na binding energy. This enhancement resulted in stronger polarization and orbital hybridization of H2 within the 2Na@BIG system compared to the 2Na@IG system, further boosting its adsorption performance. The charge transfer between Na and the substrate generated an electric field that polarized H2 adsorbed around Na, while the electric field generated by the already polarized H2 further polarizes the H2 adsorbed in the outer layer. Density of states (DOS) diagrams illustrated orbital hybridization of the H2 in both systems. Molecular dynamics simulations conducted at room temperature (300 K) demonstrated that the 2Na@BIG system achieved a hydrogen storage capacity of 8.8 wt.%. In conclusion, both 2Na@IG and 2Na@BIG systems exhibit potential as H2 storage materials, but the 2Na@BIG system displays superior hydrogen storage performance compared to the 2Na@IG system.
中文翻译:

Irida-石墨烯储氢特性的第一性原理研究
使用第一性原理计算研究了 2D 碳同素异形体 Irida-graphene (IG) 的储氢特性。H2 的本征 IG 吸附能仅为 −0.06 eV,明显低于有效吸附阈值。为了提高其储氢能力,IG 掺杂了硼 (B) 并用钠 (Na) 进行了改性。结果发现 2Na@IG 和 2Na@BIG 系统都可以吸附 8 对 H2。然而,H2 在 2Na@BIG 体系中的平均吸附能 (-0.145 eV) 高于 2Na@IG 体系 (-0.134 eV),吸附容量 (14.6 wt%) 优于 2Na@IG 体系 (14.5 wt%)。B 的引入产生了一个缺电子结构 (BIG),增强了 Na 和衬底之间的电子转移,从而提高了 Na 结合能。与 2Na@IG 系统相比,这种增强导致 H2 在 2Na@BIG 系统内的极化和轨道杂化更强,进一步提高了其吸附性能。Na 和衬底之间的电荷转移产生了一个电场,使 H2 吸附在 Na 周围,而已经极化的 H2 产生的电场进一步极化了吸附在外层的 H2。状态密度 (DOS) 图说明了 H2 在两个系统中的轨道杂交。在室温 (300 K) 下进行的分子动力学模拟表明,2Na@BIG 系统实现了 8.8 wt.% 的储氢容量。 总之,2Na@IG 和 2Na@BIG 系统都表现出作为 H2 储存材料的潜力,但与 2Na@IG 系统相比,2Na@BIG 系统表现出卓越的储氢性能。
更新日期:2024-12-06
中文翻译:

Irida-石墨烯储氢特性的第一性原理研究
使用第一性原理计算研究了 2D 碳同素异形体 Irida-graphene (IG) 的储氢特性。H2 的本征 IG 吸附能仅为 −0.06 eV,明显低于有效吸附阈值。为了提高其储氢能力,IG 掺杂了硼 (B) 并用钠 (Na) 进行了改性。结果发现 2Na@IG 和 2Na@BIG 系统都可以吸附 8 对 H2。然而,H2 在 2Na@BIG 体系中的平均吸附能 (-0.145 eV) 高于 2Na@IG 体系 (-0.134 eV),吸附容量 (14.6 wt%) 优于 2Na@IG 体系 (14.5 wt%)。B 的引入产生了一个缺电子结构 (BIG),增强了 Na 和衬底之间的电子转移,从而提高了 Na 结合能。与 2Na@IG 系统相比,这种增强导致 H2 在 2Na@BIG 系统内的极化和轨道杂化更强,进一步提高了其吸附性能。Na 和衬底之间的电荷转移产生了一个电场,使 H2 吸附在 Na 周围,而已经极化的 H2 产生的电场进一步极化了吸附在外层的 H2。状态密度 (DOS) 图说明了 H2 在两个系统中的轨道杂交。在室温 (300 K) 下进行的分子动力学模拟表明,2Na@BIG 系统实现了 8.8 wt.% 的储氢容量。 总之,2Na@IG 和 2Na@BIG 系统都表现出作为 H2 储存材料的潜力,但与 2Na@IG 系统相比,2Na@BIG 系统表现出卓越的储氢性能。






























 京公网安备 11010802027423号
京公网安备 11010802027423号