Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent advances in biotin-based therapeutic agents for cancer therapy
Nanoscale ( IF 5.8 ) Pub Date : 2024-12-06 , DOI: 10.1039/d4nr03729d Chao Wang, Yutao Xiu, Yujing Zhang, Yanhong Wang, Jiazhen Xu, Wanpeng Yu, Dongming Xing
Nanoscale ( IF 5.8 ) Pub Date : 2024-12-06 , DOI: 10.1039/d4nr03729d Chao Wang, Yutao Xiu, Yujing Zhang, Yanhong Wang, Jiazhen Xu, Wanpeng Yu, Dongming Xing
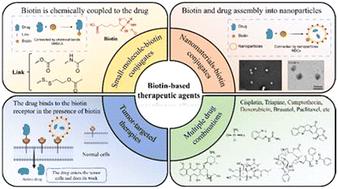
|
Biotin receptors, as biomarkers for cancer cells, are overexpressed in various tumor types. Compared to other vitamin receptors, such as folate receptors and vitamin B12 receptors, biotin receptor-based targeting strategies exhibit superior specificity and broader potential in treating aggressive cancers, including ovarian cancer, leukemia, colon cancer, breast cancer, kidney cancer, and lung cancer. These strategies promote biotin transport via receptor-mediated endocytosis, which is triggered upon ligand binding. Biotin, as the ligand of the biotin receptor, can be conjugated to anti-cancer drugs to form targeted therapies that bind to receptors overexpressed on tumor cells, thus increasing drug uptake. Despite these advantages, many candidate drugs have progressed slowly and remain in the preclinical stage, impeding clinical translation. This is mainly due to the effects of various conjugation methods and drug formulations on their functionality and efficacy. Therefore, developing novel biotin-based therapeutics is crucial. The innovation of this strategy lies in its multifunctionality—researchers can use different conjugation methods to design and synthesize these drugs, enabling precise targeting of various tumor types while minimizing toxicity to normal cells. These drugs include small-molecule-biotin conjugates (SMBCs) and nano-biotin conjugates (NBCs). This dual-platform approach represents a significant advancement in targeted therapy, offering unprecedented flexibility in drug design and delivery. Compared to chemotherapy drugs and traditional delivery systems, biotin-based drugs with tumor-specific targeting demonstrate enhanced targeting, improved efficacy, and reduced toxicity. This review examines strategies and applications for enhancing the delivery of chemotherapy drugs to cancer cells, highlighting the need for high-quality conjugates and strategies. It not only summarizes the latest progress but also provides key insights into how this emerging field could revolutionize personalized cancer treatment, especially in the context of precision medicine. Additionally, it offers perspectives on future research directions in this field.
中文翻译:

基于生物素的癌症治疗剂的最新进展
生物素受体作为癌细胞的生物标志物,在各种肿瘤类型中过表达。与其他维生素受体(如叶酸受体和维生素 B12 受体)相比,基于生物素受体的靶向策略在治疗侵袭性癌症(包括卵巢癌、白血病、结肠癌、乳腺癌、肾癌和肺癌)方面表现出卓越的特异性和更广泛的潜力。这些策略通过受体介导的内吞作用促进生物素转运,内吞作用在配体结合时触发。生物素作为生物素受体的配体,可与抗癌药物偶联形成靶向治疗,与肿瘤细胞上过表达的受体结合,从而增加药物摄取。尽管有这些优势,但许多候选药物进展缓慢,仍处于临床前阶段,阻碍了临床转化。这主要是由于各种偶联方法和药物制剂对其功能和功效的影响。因此,开发基于生物素的新型疗法至关重要。该策略的创新在于其多功能性——研究人员可以使用不同的偶联方法来设计和合成这些药物,从而能够精确靶向各种肿瘤类型,同时最大限度地减少对正常细胞的毒性。这些药物包括小分子生物素偶联物 (SMBC) 和纳米生物素偶联物 (NBC)。这种双平台方法代表了靶向治疗的重大进步,在药物设计和交付方面提供了前所未有的灵活性。与化疗药物和传统递送系统相比,具有肿瘤特异性靶向的基于生物素的药物表现出增强的靶向性、更高的疗效和更低的毒性。 本综述研究了增强化疗药物向癌细胞递送的策略和应用,强调了对高质量偶联物和策略的需求。它不仅总结了最新进展,还为这一新兴领域如何彻底改变个性化癌症治疗提供了重要见解,尤其是在精准医疗的背景下。此外,它还为该领域未来的研究方向提供了展望。
更新日期:2024-12-06
中文翻译:

基于生物素的癌症治疗剂的最新进展
生物素受体作为癌细胞的生物标志物,在各种肿瘤类型中过表达。与其他维生素受体(如叶酸受体和维生素 B12 受体)相比,基于生物素受体的靶向策略在治疗侵袭性癌症(包括卵巢癌、白血病、结肠癌、乳腺癌、肾癌和肺癌)方面表现出卓越的特异性和更广泛的潜力。这些策略通过受体介导的内吞作用促进生物素转运,内吞作用在配体结合时触发。生物素作为生物素受体的配体,可与抗癌药物偶联形成靶向治疗,与肿瘤细胞上过表达的受体结合,从而增加药物摄取。尽管有这些优势,但许多候选药物进展缓慢,仍处于临床前阶段,阻碍了临床转化。这主要是由于各种偶联方法和药物制剂对其功能和功效的影响。因此,开发基于生物素的新型疗法至关重要。该策略的创新在于其多功能性——研究人员可以使用不同的偶联方法来设计和合成这些药物,从而能够精确靶向各种肿瘤类型,同时最大限度地减少对正常细胞的毒性。这些药物包括小分子生物素偶联物 (SMBC) 和纳米生物素偶联物 (NBC)。这种双平台方法代表了靶向治疗的重大进步,在药物设计和交付方面提供了前所未有的灵活性。与化疗药物和传统递送系统相比,具有肿瘤特异性靶向的基于生物素的药物表现出增强的靶向性、更高的疗效和更低的毒性。 本综述研究了增强化疗药物向癌细胞递送的策略和应用,强调了对高质量偶联物和策略的需求。它不仅总结了最新进展,还为这一新兴领域如何彻底改变个性化癌症治疗提供了重要见解,尤其是在精准医疗的背景下。此外,它还为该领域未来的研究方向提供了展望。






























 京公网安备 11010802027423号
京公网安备 11010802027423号