当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Improved Commercial Process for the Preparation of Lifitegrast
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-12-06 , DOI: 10.1021/acs.oprd.4c00356 Arvind Girkar, Dujon Noronha, Prashant B. Patil, Mustapha Mandewale, Sudhir Sawant, Mohan Anand Chandavarkar, Kishor More
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-12-06 , DOI: 10.1021/acs.oprd.4c00356 Arvind Girkar, Dujon Noronha, Prashant B. Patil, Mustapha Mandewale, Sudhir Sawant, Mohan Anand Chandavarkar, Kishor More
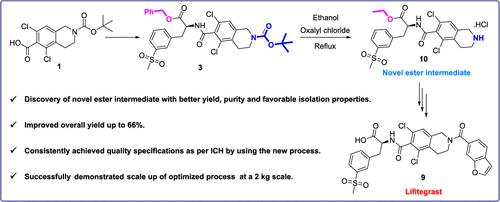
|
A straightforward, efficient, and scalable commercial manufacturing process was developed for the ophthalmic anti-inflammatory drug lifitegrast via a novel ester intermediate from commercially available starting materials. Lifitegrast (Xiidra) was approved by the FDA on July 11, 2016, for the treatment of signs and symptoms of dry eye, a syndrome called keratoconjunctivitis sicca. The breakthrough step of this new process is the discovery of an N-Boc deprotection reaction that simultaneously transesterifies an intermediate to a new ester by using oxalyl chloride, which has favorable isolation properties. As a result of transesterification, the hydrolysis of the new ester intermediate occurs under milder conditions, which improves the quality of the product by reducing racemization. Lifitegrast prepared from this new process complied with the quality guidelines, as per the International Council for Harmonization (ICH). By using this new process, lifitegrast was produced on a 2 kg scale with an overall yield of 66%.
中文翻译:

制备 Lifitegrast 的改进商业工艺
通过来自市售起始材料的新型酯中间体,为眼科抗炎药 lifitegrast 开发了一种简单、高效且可扩展的商业生产工艺。Lifitegrast (Xiidra) 于 2016 年 7 月 11 日获得 FDA 批准,用于治疗干眼症的体征和症状,这种综合征称为干燥性角膜结膜炎。这一新工艺的突破性步骤是发现了一种 N-Boc 脱保护反应,该反应通过使用草酰氯同时将中间体转酯为新酯,草酰氯具有良好的分离性能。酯交换作用的结果是,新酯中间体的水解发生在较温和的条件下,通过减少外消旋化来提高产品质量。根据国际协调委员会 (ICH) 的规定,采用这种新工艺制备的 Lifitegrast 符合质量指南。通过使用这种新工艺,lifitegrast 以 2 公斤的规模生产,总产量为 66%。
更新日期:2024-12-06
中文翻译:

制备 Lifitegrast 的改进商业工艺
通过来自市售起始材料的新型酯中间体,为眼科抗炎药 lifitegrast 开发了一种简单、高效且可扩展的商业生产工艺。Lifitegrast (Xiidra) 于 2016 年 7 月 11 日获得 FDA 批准,用于治疗干眼症的体征和症状,这种综合征称为干燥性角膜结膜炎。这一新工艺的突破性步骤是发现了一种 N-Boc 脱保护反应,该反应通过使用草酰氯同时将中间体转酯为新酯,草酰氯具有良好的分离性能。酯交换作用的结果是,新酯中间体的水解发生在较温和的条件下,通过减少外消旋化来提高产品质量。根据国际协调委员会 (ICH) 的规定,采用这种新工艺制备的 Lifitegrast 符合质量指南。通过使用这种新工艺,lifitegrast 以 2 公斤的规模生产,总产量为 66%。






























 京公网安备 11010802027423号
京公网安备 11010802027423号