当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Collaborative multi-interface engineering and dynamic iron exchange boost robust bifunctional water electrolysis at 2 A cm−2
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-12-06 , DOI: 10.1039/d4ee04619f Dongyang Li, Yong Zhang, Weiqiang Xie, Qian Zhou, Fang Yu, Ying Qi, Ziyi Lian, Long Zhang, Hui Wang, Dongsheng Tang, Haiqing Zhou
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-12-06 , DOI: 10.1039/d4ee04619f Dongyang Li, Yong Zhang, Weiqiang Xie, Qian Zhou, Fang Yu, Ying Qi, Ziyi Lian, Long Zhang, Hui Wang, Dongsheng Tang, Haiqing Zhou
|
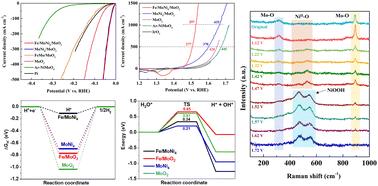
Due to the incompatibility and inconsistency of the active species for hydrogen and oxygen evolution reactions, nearly all the intermetallic catalysts present superb catalytic activity for one half reaction at the expense of another reaction activity, thus leading to large electric power consumption of alkaline water splitting. To achieve low-voltage electrochemical H2 production through water electrolysis, here we present a hierarchical trimetal hybrid catalyst comprising intermetallic nickel–molybdenum alloy (MoNi4) particles and metallic iron particles anchoring on MoO2 nanorod arrays with synergistic multimetal sites that exhibit relay catalysis for bifunctional water splitting as rationalized by operando Raman, X-ray photoelectron spectroscopic studies and density functional theory (DFT) calculations. These metal sites situated at the multilevel interfaces of Fe/MoNi4/MoO2 collaboratively promote the reaction pathway including initial water adsorption/dissociation, hydrogen adsorption and oxygen-containing intermediate adsorption, thereby substantially jeopardizing overall water splitting at 500/1000 mA cm−2 with a record low cell voltage of around 1.6 V, which is exceptionally better than that of noble IrO2(+)||Pt/C(−) couple electrodes (>1.9 V). This intermetallic catalyst demands extremely low overpotentials of 59 and 277 mV for hydrogen and oxygen evolution reactions at 500 mA cm−2, outperforming nearly all the inexpensive bifunctional electrocatalysts. Especially, this catalyst can exhibit superior catalytic performance at an industrial-level current density of 500–2000 mA cm−2 without noticeable degradation. This work paves a promising avenue to develop efficient bifunctional non-noble catalysts for industrial-level water electrolysis via relay catalysis.
中文翻译:

协作式多界面工程和动态铁交换可在 2 A cm−2 下实现稳健的双功能水电解
由于析氢和析氧反应中活性物质的不相容性和不一致性,几乎所有的金属间化合物催化剂在半反应中都表现出极好的催化活性,而牺牲了另一种反应活性,从而导致碱性水分解的电力消耗大。为了实现通过水电解产生低压电化学 H2,我们在这里提出了一种分层三金属杂化催化剂,该催化剂由金属间镍钼合金 (MoNi4) 颗粒和金属铁颗粒锚定在 MoO2 纳米棒阵列上,具有协同多金属位点,表现出双功能水分解的中继催化,由原位拉曼合理化,X 射线光电子光谱研究和密度泛函理论 (DFT) 计算。这些位于 Fe/MoNi4/MoO2 多能级界面上的金属位点协同促进反应途径,包括初始水吸附/解离、氢吸附和含氧中间吸附,从而在 500/1000 mA cm-2 时以创纪录的低电池电压约为 1.6 V 时严重危害整体水分解,这比贵金属 IrO 的电池电压要好得多阿拉伯数字(+)||Pt/C(−) 耦合电极 (>1.9 V)。在 500 mA cm-2 下,这种金属间化合物催化剂对析氢和析氧反应需要极低的 59 和 277 mV 过电位,性能几乎优于所有廉价的双功能电催化剂。 特别是,这种催化剂可以在 500–2000 mA cm-2 的工业级电流密度下表现出卓越的催化性能,而不会明显降解。这项工作为开发高效的双功能非惰性催化剂铺平了一条有前途的途径,用于通过继电器催化进行工业级水电解。
更新日期:2024-12-06
中文翻译:

协作式多界面工程和动态铁交换可在 2 A cm−2 下实现稳健的双功能水电解
由于析氢和析氧反应中活性物质的不相容性和不一致性,几乎所有的金属间化合物催化剂在半反应中都表现出极好的催化活性,而牺牲了另一种反应活性,从而导致碱性水分解的电力消耗大。为了实现通过水电解产生低压电化学 H2,我们在这里提出了一种分层三金属杂化催化剂,该催化剂由金属间镍钼合金 (MoNi4) 颗粒和金属铁颗粒锚定在 MoO2 纳米棒阵列上,具有协同多金属位点,表现出双功能水分解的中继催化,由原位拉曼合理化,X 射线光电子光谱研究和密度泛函理论 (DFT) 计算。这些位于 Fe/MoNi4/MoO2 多能级界面上的金属位点协同促进反应途径,包括初始水吸附/解离、氢吸附和含氧中间吸附,从而在 500/1000 mA cm-2 时以创纪录的低电池电压约为 1.6 V 时严重危害整体水分解,这比贵金属 IrO 的电池电压要好得多阿拉伯数字(+)||Pt/C(−) 耦合电极 (>1.9 V)。在 500 mA cm-2 下,这种金属间化合物催化剂对析氢和析氧反应需要极低的 59 和 277 mV 过电位,性能几乎优于所有廉价的双功能电催化剂。 特别是,这种催化剂可以在 500–2000 mA cm-2 的工业级电流密度下表现出卓越的催化性能,而不会明显降解。这项工作为开发高效的双功能非惰性催化剂铺平了一条有前途的途径,用于通过继电器催化进行工业级水电解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号