当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Ampicillin Hydrolysis by New Delhi Metallo‐β‐Lactamase 1: Insight From QM/MM MP2 Calculation
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-12-05 , DOI: 10.1002/jcc.27544 Rui Lai, Hui Li
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-12-05 , DOI: 10.1002/jcc.27544 Rui Lai, Hui Li
|
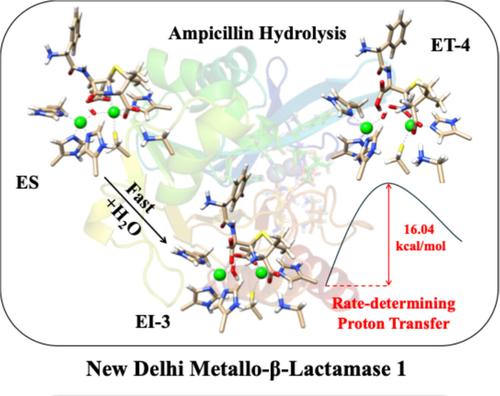
The New Delhi metallo‐β‐lactamase 1 (NDM‐1) can hydrolyze nearly all clinically important β‐lactam antibiotics, narrowing the options for effective treatment of bacterial infections. QM/MM MP2 calculations are performed to reveal the mechanism of ampicillin hydrolysis catalyzed by NDM‐1. It is found that the rate‐determining step is the dissociation of hydrolyzed ampicillin from the NDM‐1 active site, which requires a proton transfer from the bridging neutral water molecule to the newly formed carboxylate group. The precedent reaction steps, including the hydroxide nucleophilic addition, CN bond cleavage, and the protonation of the negative lactam N atom by a solvent water molecule, all require insignificant activation free energies. The calculated activation free energy for this rate‐determining proton transfer step is 16.0 kcal/mol, in good agreement with experimental values of 13.7 ~ 14.7 kcal/mol. This proton transfer step exhibits a solvent hydrogen‐deuterium kinetic isotope effect of 3.4, consistent with several experimental kinetic results.
中文翻译:

新德里金属-β-内酰胺酶 1 水解氨苄青霉素的机制:来自 QM/MM MP2 计算的见解
新德里金属-β-内酰胺酶 1 (NDM-1) 可以水解几乎所有临床上重要的 β-内酰胺类抗生素,从而缩小了有效治疗细菌感染的选择范围。进行 QM/MM MP2 计算以揭示 NDM-1 催化的氨苄青霉素水解机制。研究发现,速率确定步骤是水解氨苄青霉素从 NDM-1 活性位点解离,这需要质子从桥接中性水分子转移到新形成的羧酸盐基团。先例反应步骤,包括氢氧化物亲核加成、CN 键裂解和溶剂水分子对负内酰胺 N 原子的质子化,都需要微不足道的活化自由能。这个确定速率的质子转移步骤的计算活化自由能为 16.0 kcal/mol,与 13.7 ~ 14.7 kcal/mol 的实验值非常一致。该质子转移步骤表现出 3.4 的溶剂氢-氘动力学同位素效应,与几个实验动力学结果一致。
更新日期:2024-12-05
中文翻译:

新德里金属-β-内酰胺酶 1 水解氨苄青霉素的机制:来自 QM/MM MP2 计算的见解
新德里金属-β-内酰胺酶 1 (NDM-1) 可以水解几乎所有临床上重要的 β-内酰胺类抗生素,从而缩小了有效治疗细菌感染的选择范围。进行 QM/MM MP2 计算以揭示 NDM-1 催化的氨苄青霉素水解机制。研究发现,速率确定步骤是水解氨苄青霉素从 NDM-1 活性位点解离,这需要质子从桥接中性水分子转移到新形成的羧酸盐基团。先例反应步骤,包括氢氧化物亲核加成、CN 键裂解和溶剂水分子对负内酰胺 N 原子的质子化,都需要微不足道的活化自由能。这个确定速率的质子转移步骤的计算活化自由能为 16.0 kcal/mol,与 13.7 ~ 14.7 kcal/mol 的实验值非常一致。该质子转移步骤表现出 3.4 的溶剂氢-氘动力学同位素效应,与几个实验动力学结果一致。































 京公网安备 11010802027423号
京公网安备 11010802027423号