当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Humic acid promotes pathway of chloronitrobenzene cathodic reduction shunting from atomic H* to direct electron transfer
Water Research ( IF 11.4 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.watres.2024.122918 Caiqin Wang, Hangzhe Chen, Yunjie Zhou, Tao Wen, Junjie Shao, Daoyong Zhang, Xiangliang Pan
Water Research ( IF 11.4 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.watres.2024.122918 Caiqin Wang, Hangzhe Chen, Yunjie Zhou, Tao Wen, Junjie Shao, Daoyong Zhang, Xiangliang Pan
|
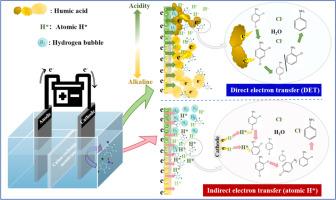
Progress mechanism of humic acid (HA) interacting with chloronitrobenzenes (ClNBs) and affecting their reduction and degradation was investigated. A two-stage chamber with graphite felt as cathode and anode was constructed, and 2,4-dichloronitrobenzene (2,4-DCNB) was target pollutant. Result showed that HA increased 36.15 % of 2,4-DCNB removal efficiency at pH 5.0 after 4-h cathodic reduction, while at pH 7.0 and 10.0, it showed much less effect. Meanwhile, HA reduced cathodic H2 and atomic H* production via competing electron with H + . Combined with result of HA alleviating inhibition of tert‑butyl alcohol on 2,4-DCNB removal, it was supposed that HA shunted dominant pathway of cathodic reduction of 2,4-DCNB from atomic H* to direct electron transfer (DET). This was proved by HA increasing electron transfer efficiency (k ) of 2,4-DCNB reduction from 0.50±0.01 to 0.63±0.01, which indicated rate-limiting step of 2,4-DCNB reduction changed from electron transfer (ET) to bond breaking kinetics. HA-mediated DET pathway initially reduced nitro group, followed by dechlorination, while atomic H* pathway was randomly dechlorinated and nitro reduced. The pH significantly affected agglomeration of HA and 2,4-DCNB. Molecular dynamics simulation showed that hydrogen bond and Van der Waals force dominated agglomeration of HA and 2,4-DCNB in acidic condition, while electrostatic force was main driving force in alkaline condition. Less effect of HA on 2,4-DCNB removal efficiency at high pH could be related to its reduced conductivity and weaker molecular interactions with 2,4-DCNB. This study provides comprehensive insight into role and impact of HA in remediation of ClNBs-contaminated sediment and water by (bio)electrochemical technology.
中文翻译:

腐植酸促进氯硝基苯阴极还原途径从原子 H* 分流到直接电子转移
研究了腐植酸 (HA) 与氯硝基苯 (ClNBs) 相互作用及其还原降解的进展机制。构建了一个以石墨毡为阴极和阳极的两级腔室,2,4-二氯硝基苯 (2,4-DCNB) 是目标污染物。结果表明,阴极还原 4 小时后,HA 在 pH 5.0 下提高了 36.15% 的 2,4-DCNB 去除效率,而在 pH 7.0 和 10.0 下,效果要小得多。同时,HA 通过与 H+ 竞争电子减少了阴极 H2 和原子 H* 的产生。结合 HA 减轻叔丁醇对 2,4-DCNB 去除的抑制的结果,假设 HA 将 2,4-DCNB 阴极还原的主要途径从原子 H* 分流到直接电子转移 (DET)。HA 将 2,4-DCNB 还原的电子转移效率 (k) 从 0.50±0.01 提高到 0.63±0.01 证明了这一点,这表明 2,4-DCNB 还原的限速步骤从电子转移 (ET) 变为键断裂动力学。HA 介导的 DET 途径最初还原硝基,然后是脱氯,而原子 H* 途径是随机脱氯和硝基还原。pH 值显著影响 HA 和 2,4-DCNB 的团聚。分子动力学模拟表明,在酸性条件下,氢键和范德华力主导了 HA 和 2,4-DCNB 的团聚,而在碱性条件下,静电力是主要驱动力。在高 pH 值下,HA 对 2,4-DCNB 去除效率的影响较小,这可能与其电导率降低和与 2,4-DCNB 的分子相互作用较弱有关。本研究全面介绍了 HA 在通过(生物)电化学技术修复 ClNBs 污染的沉积物和水中的作用和影响。
更新日期:2024-12-04
中文翻译:

腐植酸促进氯硝基苯阴极还原途径从原子 H* 分流到直接电子转移
研究了腐植酸 (HA) 与氯硝基苯 (ClNBs) 相互作用及其还原降解的进展机制。构建了一个以石墨毡为阴极和阳极的两级腔室,2,4-二氯硝基苯 (2,4-DCNB) 是目标污染物。结果表明,阴极还原 4 小时后,HA 在 pH 5.0 下提高了 36.15% 的 2,4-DCNB 去除效率,而在 pH 7.0 和 10.0 下,效果要小得多。同时,HA 通过与 H+ 竞争电子减少了阴极 H2 和原子 H* 的产生。结合 HA 减轻叔丁醇对 2,4-DCNB 去除的抑制的结果,假设 HA 将 2,4-DCNB 阴极还原的主要途径从原子 H* 分流到直接电子转移 (DET)。HA 将 2,4-DCNB 还原的电子转移效率 (k) 从 0.50±0.01 提高到 0.63±0.01 证明了这一点,这表明 2,4-DCNB 还原的限速步骤从电子转移 (ET) 变为键断裂动力学。HA 介导的 DET 途径最初还原硝基,然后是脱氯,而原子 H* 途径是随机脱氯和硝基还原。pH 值显著影响 HA 和 2,4-DCNB 的团聚。分子动力学模拟表明,在酸性条件下,氢键和范德华力主导了 HA 和 2,4-DCNB 的团聚,而在碱性条件下,静电力是主要驱动力。在高 pH 值下,HA 对 2,4-DCNB 去除效率的影响较小,这可能与其电导率降低和与 2,4-DCNB 的分子相互作用较弱有关。本研究全面介绍了 HA 在通过(生物)电化学技术修复 ClNBs 污染的沉积物和水中的作用和影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号