当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An orbital-overlap complement to σ-hole electrostatic potentials
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-05 , DOI: 10.1039/d4cp03851g Arshad Mehmood, Benjamin G. Janesko
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-05 , DOI: 10.1039/d4cp03851g Arshad Mehmood, Benjamin G. Janesko
|
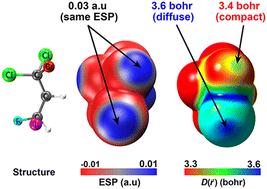
A σ-hole is an electron-deficient region of positive electrostatic potential (ESP) opposite from a half-filled p orbital involved in forming a covalent bond. The σ-hole concept helps rationalize directional noncovalent interactions, known as σ-hole bonds, between covalently bonded group V–VII atoms and electron-pair donors. The magnitude and orientation of σ-holes are correlated with the strength and geometry of halogen bonds. However, ESP computed for isolated σ-holes are not always predictive of interaction energies. For example, the σ-holes of isolated CHFBr2 and isolated CH2FI have identical ESP on the molecule surface, but halogen bonds to these molecules generally have different strengths. Here we show that the compact/diffuse nature of the orbitals involved plays an important role. Our orbital overlap distance quantifies the compact/diffuse nature of the “test orbital” that best overlaps with a systems orbitals at each point. The overlap distance captures the response properties of σ-holes: diffuse σ-holes with large overlap distance are typically “softer” and more polarizable. This aids visualization and interpretation. A linear fit to overlap distance and ESP is predictive of the halogen bond strengths of CH3X and CF3X (X = Cl, Br and I). We suggest that the overlap distance will be a useful partner to ESP for characterizing σ-holes.
中文翻译:

σ 空穴静电电位的轨道重叠补充
σ 空穴是正静电势 (ESP) 的缺电子区域,与参与形成共价键的半填充 p 轨道相对。σ 空穴概念有助于合理化共价键合的 V-VII 基团原子和电子对供体之间的定向非共价相互作用,称为σ孔键。σ 孔的大小和方向与卤素键的强度和几何形状相关。然而,为孤立的 σ 孔计算的 ESP 并不总是能预测相互作用能。例如,孤立的 CHFBr2 和孤立的 CH2FI 的σ孔在分子表面具有相同的 ESP,但与这些分子的卤素键通常具有不同的强度。在这里,我们表明所涉及的轨道的致密/弥散性质起着重要作用。我们的轨道重叠距离量化了“测试轨道”的紧凑/弥散性质,该轨道在每个点上与系统轨道最重叠。重叠距离捕获了σ孔的响应特性:具有较大重叠距离的漫射σ孔通常“更软”且更容易极化。这有助于可视化和解释。与重叠距离和 ESP 的线性拟合可预测 CH3X 和 CF3X 的卤素键强度(X = Cl、Br 和 I)。我们建议重叠距离将成为 ESP 表征σ孔的有用伙伴。
更新日期:2024-12-05
中文翻译:

σ 空穴静电电位的轨道重叠补充
σ 空穴是正静电势 (ESP) 的缺电子区域,与参与形成共价键的半填充 p 轨道相对。σ 空穴概念有助于合理化共价键合的 V-VII 基团原子和电子对供体之间的定向非共价相互作用,称为σ孔键。σ 孔的大小和方向与卤素键的强度和几何形状相关。然而,为孤立的 σ 孔计算的 ESP 并不总是能预测相互作用能。例如,孤立的 CHFBr2 和孤立的 CH2FI 的σ孔在分子表面具有相同的 ESP,但与这些分子的卤素键通常具有不同的强度。在这里,我们表明所涉及的轨道的致密/弥散性质起着重要作用。我们的轨道重叠距离量化了“测试轨道”的紧凑/弥散性质,该轨道在每个点上与系统轨道最重叠。重叠距离捕获了σ孔的响应特性:具有较大重叠距离的漫射σ孔通常“更软”且更容易极化。这有助于可视化和解释。与重叠距离和 ESP 的线性拟合可预测 CH3X 和 CF3X 的卤素键强度(X = Cl、Br 和 I)。我们建议重叠距离将成为 ESP 表征σ孔的有用伙伴。






























 京公网安备 11010802027423号
京公网安备 11010802027423号