当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Urea hydrogen-bond donor strengths: bigger is not always better
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-05 , DOI: 10.1039/d4cp04042b Celine Nieuwland, Angelina N. van Dam, F. Matthias Bickelhaupt, Célia Fonseca Guerra
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-12-05 , DOI: 10.1039/d4cp04042b Celine Nieuwland, Angelina N. van Dam, F. Matthias Bickelhaupt, Célia Fonseca Guerra
|
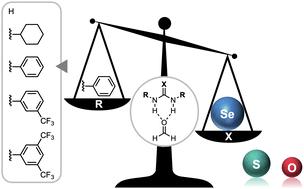
The hydrogen-bond donor strength of ureas, widely used in hydrogen-bond donor catalysis, molecular recognition, and self-assembly, can be enhanced by increasing the size of the chalcogen X in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X bond from O to S to Se and by introducing more electron-withdrawing substituents because both modifications increase the positive charge on the NH groups which become better hydrogen-bond donors. However, in 1,3-diaryl X-ureas, a steric mechanism disrupts the positive additivity of these two tuning factors, as revealed by our quantum-chemical analyses. This leads to an enhanced hydrogen-bond donor strength, despite a lower NH acidity, for 1,3-diaryl substituted O-ureas compared to the S- and Se-urea analogs. In addition, we provide a strategy to overcome this steric limitation using a predistorted urea-type hydrogen-bond donor featuring group 14 elements in the C
X bond from O to S to Se and by introducing more electron-withdrawing substituents because both modifications increase the positive charge on the NH groups which become better hydrogen-bond donors. However, in 1,3-diaryl X-ureas, a steric mechanism disrupts the positive additivity of these two tuning factors, as revealed by our quantum-chemical analyses. This leads to an enhanced hydrogen-bond donor strength, despite a lower NH acidity, for 1,3-diaryl substituted O-ureas compared to the S- and Se-urea analogs. In addition, we provide a strategy to overcome this steric limitation using a predistorted urea-type hydrogen-bond donor featuring group 14 elements in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X bond so that the hydrogen-bond donor strength of X-urea derivatives bearing two aryl substituents can be enhanced upon varying X down group 14.
X bond so that the hydrogen-bond donor strength of X-urea derivatives bearing two aryl substituents can be enhanced upon varying X down group 14.
中文翻译:

尿素氢键供体优势:越大并不总是越好
广泛用于氢键供体催化、分子识别和自组装的尿素的氢键供体强度可以通过将 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X 键中硫属元 X 的大小从 O 增加到 S 再到 Se 并引入更多的吸电子取代基来提高,因为这两种修饰都增加了 NH 基团上的正电荷,从而成为更好的氢键供体。然而,正如我们的量子化学分析所揭示的那样,在 1,3-二芳基 X-脲中,空间机制破坏了这两个调谐因子的正加性。与 S 和硒尿素类似物相比,尽管 NH 酸度较低,但这导致 1,3-二芳基取代的 O-脲的氢键供体强度增强。此外,我们提供了一种策略来克服这种空间限制,使用在 C
X 键中硫属元 X 的大小从 O 增加到 S 再到 Se 并引入更多的吸电子取代基来提高,因为这两种修饰都增加了 NH 基团上的正电荷,从而成为更好的氢键供体。然而,正如我们的量子化学分析所揭示的那样,在 1,3-二芳基 X-脲中,空间机制破坏了这两个调谐因子的正加性。与 S 和硒尿素类似物相比,尽管 NH 酸度较低,但这导致 1,3-二芳基取代的 O-脲的氢键供体强度增强。此外,我们提供了一种策略来克服这种空间限制,使用在 C ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X 键中具有第 14 族元素的预扭曲尿素型氢键供体,以便在改变 X 向下第 14 族时可以提高带有两个芳基取代基的 X-尿素衍生物的氢键供体强度。
X 键中具有第 14 族元素的预扭曲尿素型氢键供体,以便在改变 X 向下第 14 族时可以提高带有两个芳基取代基的 X-尿素衍生物的氢键供体强度。
更新日期:2024-12-05
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X bond from O to S to Se and by introducing more electron-withdrawing substituents because both modifications increase the positive charge on the NH groups which become better hydrogen-bond donors. However, in 1,3-diaryl X-ureas, a steric mechanism disrupts the positive additivity of these two tuning factors, as revealed by our quantum-chemical analyses. This leads to an enhanced hydrogen-bond donor strength, despite a lower NH acidity, for 1,3-diaryl substituted O-ureas compared to the S- and Se-urea analogs. In addition, we provide a strategy to overcome this steric limitation using a predistorted urea-type hydrogen-bond donor featuring group 14 elements in the C
X bond from O to S to Se and by introducing more electron-withdrawing substituents because both modifications increase the positive charge on the NH groups which become better hydrogen-bond donors. However, in 1,3-diaryl X-ureas, a steric mechanism disrupts the positive additivity of these two tuning factors, as revealed by our quantum-chemical analyses. This leads to an enhanced hydrogen-bond donor strength, despite a lower NH acidity, for 1,3-diaryl substituted O-ureas compared to the S- and Se-urea analogs. In addition, we provide a strategy to overcome this steric limitation using a predistorted urea-type hydrogen-bond donor featuring group 14 elements in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X bond so that the hydrogen-bond donor strength of X-urea derivatives bearing two aryl substituents can be enhanced upon varying X down group 14.
X bond so that the hydrogen-bond donor strength of X-urea derivatives bearing two aryl substituents can be enhanced upon varying X down group 14.
中文翻译:

尿素氢键供体优势:越大并不总是越好
广泛用于氢键供体催化、分子识别和自组装的尿素的氢键供体强度可以通过将 C






























 京公网安备 11010802027423号
京公网安备 11010802027423号