当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-light-induced radical C(sp3)–S coupling for the synthesis of cyanoalkyl thioethers
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-05 , DOI: 10.1039/d4qo01994f Zhi-Qiang Zhu, Xiao-Wen Zheng, Dong-Liang Zhang, Xiao-Long Huang, Qian-Qian Xu, Zong-Bo Xie, Zhang-Gao Le
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-05 , DOI: 10.1039/d4qo01994f Zhi-Qiang Zhu, Xiao-Wen Zheng, Dong-Liang Zhang, Xiao-Long Huang, Qian-Qian Xu, Zong-Bo Xie, Zhang-Gao Le
|
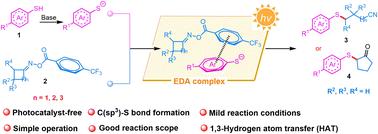
Herein, visible-light-driven radical–radical cross-coupling for the construction of a C(sp3)–S bond to afford various cyanoalkyl thioethers through EDA complex-promoted C–C and C–H bond cleavage is described. The photoactivation of a transiently assembled chromophore between thiolate anions and cycloketone oxime esters is critical to the generation of the C(sp3)–S bond. Notably, we also realize the in situ formed EDA complex-mediated 1,3-hydrogen atom transfer (HAT) to construct diverse thioethers under the standard conditions. This synthetic method features easily available substrates, a wide substrate scope, and mild reaction conditions, and has potential to be used for the preparation of valuable sulfides.
中文翻译:

可见光诱导的自由基 C(sp3)-S 偶联物联用于合成氰烷基硫醚
在此,描述了可见光驱动的自由基-自由基交叉偶联,用于构建 C(sp3)-S 键,以通过 EDA 复合物促进的 C-C 和 C-H 键裂解得到各种氰基烷基硫醚。硫代阴离子和环酮肟酯之间瞬时组装的发色团的光活化对于 C(sp3)-S 键的产生至关重要。值得注意的是,我们还实现了原位形成的 EDA 复合物介导的 1,3-氢原子转移 (HAT),以在标准条件下构建多种硫醚。这种合成方法具有易于获得的底物、广泛的底物范围和温和的反应条件,并有可能用于制备有价值的硫化物。
更新日期:2024-12-10
中文翻译:

可见光诱导的自由基 C(sp3)-S 偶联物联用于合成氰烷基硫醚
在此,描述了可见光驱动的自由基-自由基交叉偶联,用于构建 C(sp3)-S 键,以通过 EDA 复合物促进的 C-C 和 C-H 键裂解得到各种氰基烷基硫醚。硫代阴离子和环酮肟酯之间瞬时组装的发色团的光活化对于 C(sp3)-S 键的产生至关重要。值得注意的是,我们还实现了原位形成的 EDA 复合物介导的 1,3-氢原子转移 (HAT),以在标准条件下构建多种硫醚。这种合成方法具有易于获得的底物、广泛的底物范围和温和的反应条件,并有可能用于制备有价值的硫化物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号