Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic and structural insights into EstS1 esterase: A potent broad-spectrum phthalate diester degrading enzyme
Structure ( IF 4.4 ) Pub Date : 2024-12-05 , DOI: 10.1016/j.str.2024.11.006 Shalja Verma, Shweta Choudhary, Kamble Amith Kumar, Jai Krishna Mahto, Anil Kumar Vamsi K, Ishani Mishra, Vellanki Bhanu Prakash, Debabrata Sircar, Shailly Tomar, Ashwani Kumar Sharma, Jitin Singla, Pravindra Kumar
Structure ( IF 4.4 ) Pub Date : 2024-12-05 , DOI: 10.1016/j.str.2024.11.006 Shalja Verma, Shweta Choudhary, Kamble Amith Kumar, Jai Krishna Mahto, Anil Kumar Vamsi K, Ishani Mishra, Vellanki Bhanu Prakash, Debabrata Sircar, Shailly Tomar, Ashwani Kumar Sharma, Jitin Singla, Pravindra Kumar
|
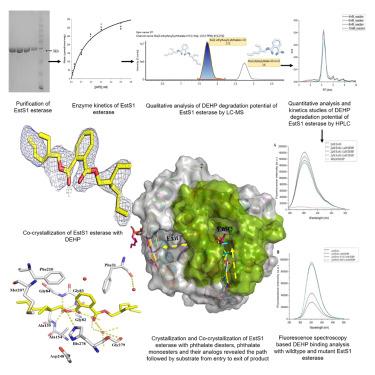
Phthalate diesters are important pollutants and act as endocrine disruptors. While certain bacterial esterases have been identified for phthalate diesters degradation to monoesters, their structural and mechanistic characteristics remain largely unexplored. Here, we highlight the potential of the thermostable and pH-tolerant EstS1 esterase from Sulfobacillus acidophilus DSM10332 to degrade high molecular weight bis(2-ethylhexyl) phthalate (DEHP) by combining biophysical and biochemical approaches along with high-resolution EstS1 crystal structures of the apo form and with bound substrates, products, and their analogs to elucidate its mechanism. The catalytic tunnel mediates entry and exit of the substrate and product, respectively. The centralized Ser-His-Asp triad performs catalysis by a bi-bi ping-pong mechanism, forming a tetrahedral intermediate. Mutagenesis analysis showed that the Met207Ala mutation abolished DEHP binding at the active site, confirming its essential role in supporting catalysis. These findings underscore EstS1 as a promising tool for advancing technologies aimed at phthalate diesters biodegradation.
中文翻译:

EstS1 酯酶的机制和结构见解:一种有效的广谱邻苯二甲酸酯二酯降解酶
邻苯二甲酸酯二酯是重要的污染物,是内分泌干扰物。虽然已经鉴定出某些细菌酯酶可用于邻苯二甲酸酯二酯降解为单酯,但它们的结构和机械特性在很大程度上仍未得到探索。在这里,我们强调了来自嗜酸磺芽孢杆菌的热稳定且耐 pH 值的 EstS1 酯酶的潜力DSM10332,通过结合生物物理和生化方法以及载脂蛋白形式的高分辨率 EstS1 晶体结构以及结合的底物、产物及其类似物来阐明其机制。催化隧道分别介导底物和产物的进入和退出。集中式 Ser-His-Asp 三元组通过 bi-bi 乒乓机制进行催化,形成四面体中间体。诱变分析表明,Met207Ala 突变消除了活性位点的 DEHP 结合,证实了其在支持催化作用中的重要作用。这些发现强调 EstS1 是推进针对邻苯二甲酸酯二酯生物降解的技术的有前途的工具。
更新日期:2024-12-05
中文翻译:

EstS1 酯酶的机制和结构见解:一种有效的广谱邻苯二甲酸酯二酯降解酶
邻苯二甲酸酯二酯是重要的污染物,是内分泌干扰物。虽然已经鉴定出某些细菌酯酶可用于邻苯二甲酸酯二酯降解为单酯,但它们的结构和机械特性在很大程度上仍未得到探索。在这里,我们强调了来自嗜酸磺芽孢杆菌的热稳定且耐 pH 值的 EstS1 酯酶的潜力DSM10332,通过结合生物物理和生化方法以及载脂蛋白形式的高分辨率 EstS1 晶体结构以及结合的底物、产物及其类似物来阐明其机制。催化隧道分别介导底物和产物的进入和退出。集中式 Ser-His-Asp 三元组通过 bi-bi 乒乓机制进行催化,形成四面体中间体。诱变分析表明,Met207Ala 突变消除了活性位点的 DEHP 结合,证实了其在支持催化作用中的重要作用。这些发现强调 EstS1 是推进针对邻苯二甲酸酯二酯生物降解的技术的有前途的工具。

































 京公网安备 11010802027423号
京公网安备 11010802027423号