当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The GIP receptor activates futile calcium cycling in white adipose tissue to increase energy expenditure and drive weight loss in mice
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-12-05 , DOI: 10.1016/j.cmet.2024.11.003 Xinxin Yu, Shiuhwei Chen, Jan-Bernd Funcke, Leon G. Straub, Valentina Pirro, Margo P. Emont, Brian A. Droz, Kyla AI. Collins, Chanmin Joung, Mackenzie J. Pearson, Corey M. James, Gopal J. Babu, Vissarion Efthymiou, Ashley Vernon, Mary Elizabeth Patti, Yu A. An, Evan D. Rosen, Matthew P. Coghlan, Ricardo J. Samms, Philipp E. Scherer, Christine M. Kusminski
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-12-05 , DOI: 10.1016/j.cmet.2024.11.003 Xinxin Yu, Shiuhwei Chen, Jan-Bernd Funcke, Leon G. Straub, Valentina Pirro, Margo P. Emont, Brian A. Droz, Kyla AI. Collins, Chanmin Joung, Mackenzie J. Pearson, Corey M. James, Gopal J. Babu, Vissarion Efthymiou, Ashley Vernon, Mary Elizabeth Patti, Yu A. An, Evan D. Rosen, Matthew P. Coghlan, Ricardo J. Samms, Philipp E. Scherer, Christine M. Kusminski
|
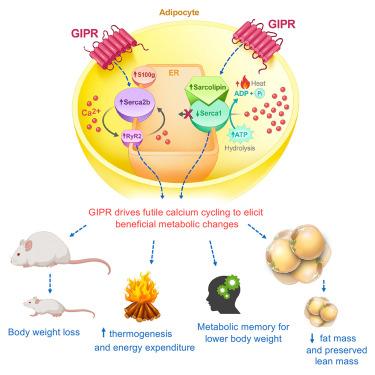
Obesity is a chronic disease that contributes to the development of insulin resistance, type 2 diabetes (T2D), and cardiovascular risk. Glucose-dependent insulinotropic polypeptide (GIP) receptor (GIPR) and glucagon-like peptide-1 (GLP-1) receptor (GLP-1R) co-agonism provide an improved therapeutic profile in individuals with T2D and obesity when compared with selective GLP-1R agonism. Although the metabolic benefits of GLP-1R agonism are established, whether GIPR activation impacts weight loss through peripheral mechanisms is yet to be fully defined. Here, we generated a mouse model of GIPR induction exclusively in the adipocyte. We show that GIPR induction in the fat cell protects mice from diet-induced obesity and triggers profound weight loss (∼35%) in an obese setting. Adipose GIPR further increases lipid oxidation, thermogenesis, and energy expenditure. Mechanistically, we demonstrate that GIPR induction activates SERCA-mediated futile calcium cycling in the adipocyte. GIPR activation further triggers a metabolic memory effect, which maintains weight loss after the transgene has been switched off, highlighting a unique aspect in adipocyte biology. Collectively, we present a mechanism of peripheral GIPR action in adipose tissue, which exerts beneficial metabolic effects on body weight and energy balance.
中文翻译:

GIP 受体激活白色脂肪组织中徒劳的钙循环,以增加能量消耗并驱动小鼠体重减轻
肥胖是一种慢性疾病,会导致胰岛素抵抗、2 型糖尿病 (T2D) 和心血管风险的发展。与选择性 GLP-1R 激动剂相比,葡萄糖依赖性促胰岛素多肽 (GIP) 受体 (GIPR) 和胰高血糖素样肽-1 (GLP-1) 受体 (GLP-1R) 共激动剂为 T2D 和肥胖症患者提供了更好的治疗效果。尽管 GLP-1R 激动作用的代谢益处已确定,但 GIPR 激活是否通过外周机制影响体重减轻尚未完全定义。在这里,我们生成了一个仅在脂肪细胞中诱导 GIPR 的小鼠模型。我们表明,脂肪细胞中的 GIPR 诱导可保护小鼠免受饮食诱导的肥胖,并在肥胖环境中触发严重的体重减轻 (∼35%)。脂肪 GIPR 进一步增加脂质氧化、产热和能量消耗。从机制上讲,我们证明 GIPR 诱导激活脂肪细胞中 SERCA 介导的徒劳钙循环。GIPR 激活进一步触发代谢记忆效应,在转基因关闭后维持体重减轻,突出了脂肪细胞生物学的一个独特方面。总的来说,我们提出了一种外周 GIPR 在脂肪组织中起作用的机制,它对体重和能量平衡产生有益的代谢影响。
更新日期:2024-12-05
中文翻译:

GIP 受体激活白色脂肪组织中徒劳的钙循环,以增加能量消耗并驱动小鼠体重减轻
肥胖是一种慢性疾病,会导致胰岛素抵抗、2 型糖尿病 (T2D) 和心血管风险的发展。与选择性 GLP-1R 激动剂相比,葡萄糖依赖性促胰岛素多肽 (GIP) 受体 (GIPR) 和胰高血糖素样肽-1 (GLP-1) 受体 (GLP-1R) 共激动剂为 T2D 和肥胖症患者提供了更好的治疗效果。尽管 GLP-1R 激动作用的代谢益处已确定,但 GIPR 激活是否通过外周机制影响体重减轻尚未完全定义。在这里,我们生成了一个仅在脂肪细胞中诱导 GIPR 的小鼠模型。我们表明,脂肪细胞中的 GIPR 诱导可保护小鼠免受饮食诱导的肥胖,并在肥胖环境中触发严重的体重减轻 (∼35%)。脂肪 GIPR 进一步增加脂质氧化、产热和能量消耗。从机制上讲,我们证明 GIPR 诱导激活脂肪细胞中 SERCA 介导的徒劳钙循环。GIPR 激活进一步触发代谢记忆效应,在转基因关闭后维持体重减轻,突出了脂肪细胞生物学的一个独特方面。总的来说,我们提出了一种外周 GIPR 在脂肪组织中起作用的机制,它对体重和能量平衡产生有益的代谢影响。






























 京公网安备 11010802027423号
京公网安备 11010802027423号