当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integrating kinetic modeling and experimental insights: PFAS electrochemical degradation in concentrated streams with a focus on organic and inorganic effects
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136624 Fatemeh Asadi Zeidabadi, Pezhman Abbasi, Ehsan Banayan Esfahani, Madjid Mohseni
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136624 Fatemeh Asadi Zeidabadi, Pezhman Abbasi, Ehsan Banayan Esfahani, Madjid Mohseni
|
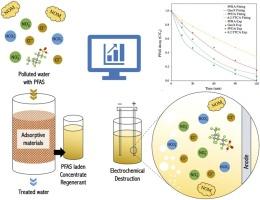
This study investigated the impact of organic and inorganic constituents on electrochemical degradation of per- and poly-fluoroalkyl substances (PFAS) in a sulfate-based brine from regeneration of spent ion exchange (IX) resin. The system's performance was assessed in the presence of natural organic matter (NOM) and common inorganic constituents: chloride, nitrate, and bicarbonate. Results revealed distinct outcomes based on constituent type, concentration, and specific PFAS variant. NOM hindered PFAS decomposition, especially for more hydrophobic compounds. Chloride reduced degradation and defluorination efficiencies through competitive interactions with PFAS for the anode’s active sites and scavenging effects on SO4 •− and • OH. Nitrate and bicarbonate minimally impacted degradation but significantly reduced defluorination. Investigating the electrochemical process in real brine solutions showed higher efficiency and lower electrical energy consumption when methanol was distilled, as methanol scavenges reactive radicals and competes for active anode sites. A kinetic model was also developed to determine the direct electron transfer (DET) and mass transfer coefficients for the species present, considering both surface and bulk solution interactions. The model predicted mass transfer (mol m−2 s−1 ) and DET (m2 mol−1 s−1 ) coefficients of 6:2 FTCA, PFOA, GenX, and PFBA to be (5.0 ×10−10 , 3.7 ×1011 ), (1.0 ×10−9 , 8.0 ×108 ), (6.0 ×10−8 , 7.5 ×108 ), and (6.2 ×10−8 , 4.2 ×108 ), respectively.
中文翻译:

整合动力学建模和实验见解:浓缩流中的 PFAS 电化学降解,重点关注有机和无机效应
本研究调查了有机和无机成分对废离子交换 (IX) 树脂再生硫酸盐基盐水中全氟烷基和多氟烷基物质 (PFAS) 电化学降解的影响。在天然有机物 (NOM) 和常见无机成分(氯化物、硝酸盐和碳酸氢盐)存在下评估系统的性能。结果揭示了基于成分类型、浓度和特异性 PFAS 变体的不同结果。NOM 阻碍了 PFAS 的分解,尤其是对于疏水性更强的化合物。氯化物通过与 PFAS 对阳极活性位点的竞争性相互作用以及对 SO4•− 和 •OH 的清除作用,降低了降解和脱氟效率。硝酸盐和碳酸氢盐对降解的影响最小,但显着减少了脱氟。研究实际盐水溶液中的电化学过程表明,蒸馏甲醇时效率更高,电能消耗更低,因为甲醇会清除反应自由基并竞争活性阳极位点。还开发了一个动力学模型来确定存在物质的直接电子转移 (DET) 和传质系数,同时考虑表面和本体溶液的相互作用。该模型预测 6:2 FTCA、PFOA、GenX 和 PFBA 的质量传递 (mol m−2 s−1) 和 DET (m2 mol−1 s−1) 系数分别为 (5.0 ×10−10, 3.7 ×1011)、(1.0 ×10−9, 8.0 ×108)、(6.0 ×10−8, 7.5 ×108) 和 (6.2 ×10−8, 4.2 ×108)。
更新日期:2024-12-04
中文翻译:

整合动力学建模和实验见解:浓缩流中的 PFAS 电化学降解,重点关注有机和无机效应
本研究调查了有机和无机成分对废离子交换 (IX) 树脂再生硫酸盐基盐水中全氟烷基和多氟烷基物质 (PFAS) 电化学降解的影响。在天然有机物 (NOM) 和常见无机成分(氯化物、硝酸盐和碳酸氢盐)存在下评估系统的性能。结果揭示了基于成分类型、浓度和特异性 PFAS 变体的不同结果。NOM 阻碍了 PFAS 的分解,尤其是对于疏水性更强的化合物。氯化物通过与 PFAS 对阳极活性位点的竞争性相互作用以及对 SO4•− 和 •OH 的清除作用,降低了降解和脱氟效率。硝酸盐和碳酸氢盐对降解的影响最小,但显着减少了脱氟。研究实际盐水溶液中的电化学过程表明,蒸馏甲醇时效率更高,电能消耗更低,因为甲醇会清除反应自由基并竞争活性阳极位点。还开发了一个动力学模型来确定存在物质的直接电子转移 (DET) 和传质系数,同时考虑表面和本体溶液的相互作用。该模型预测 6:2 FTCA、PFOA、GenX 和 PFBA 的质量传递 (mol m−2 s−1) 和 DET (m2 mol−1 s−1) 系数分别为 (5.0 ×10−10, 3.7 ×1011)、(1.0 ×10−9, 8.0 ×108)、(6.0 ×10−8, 7.5 ×108) 和 (6.2 ×10−8, 4.2 ×108)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号