当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of atomic coordination on the activity of lattice oxygen and catalytic oxidation of toluene over regular Cu2O crystalline
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136796 Linlin Deng, Mingtai Li, Xin Gao, Xiaokun Yi, Yang Zhao, Yulong Yang, Zitong Zhao, Jiarui Chen, Baojuan Dou, Feng Bin
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136796 Linlin Deng, Mingtai Li, Xin Gao, Xiaokun Yi, Yang Zhao, Yulong Yang, Zitong Zhao, Jiarui Chen, Baojuan Dou, Feng Bin
|
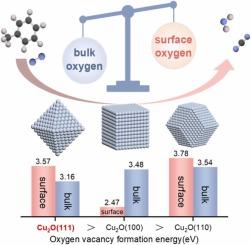
VOCs oxidation over transition metal catalyst is commonly understood via the Mars-van Krevelen mechanism involving the crucial role of lattice oxygen (OL ) activity, however, how it is influenced by atomic coordination is still unclear. Herein, we use model catalysts of Cu2 O-cub, Cu2 O-oct and Cu2 O-dod with crystal planes of (100), (111) and (110), respectively, to investigate the OL activity and catalytic oxidation of toluene. The activity of Cu2 O-oct is found to be the highest, followed by Cu2 O-cub and Cu2 O-dod. Experiments results combined with density functional theory show that, although low di-coordinated O atoms leads to the lowest surface oxygen vacancy formation energy (2.47 eV) and the highest surface OL activity of Cu2 O-cub, it cannot determine the activity. The lowest bulk oxygen vacancy formation energy (3.16 eV) in Cu2 O-oct terminated with tri-coordinated O atoms and open surface can accelerate the migration and replenishment of OL , thereby promoting the catalytic activity.
中文翻译:

原子配位对晶格氧活性和甲苯对常规 Cu2O 晶体催化氧化的影响
通常通过涉及晶格氧 (OL) 活性的关键作用的 Mars-van Krevelen 机制来理解过渡金属催化剂上的 VOCs 氧化,但是,它如何受到原子配位的影响仍不清楚。在此,我们使用晶面分别为 (100)、(111) 和 (110) 的 Cu2O-cub、Cu2O-oct 和 Cu2O-dod 模型催化剂来研究甲苯的 OL 活性和催化氧化。发现 Cu2O-oct 的活性最高,其次是 Cu2O-cub 和 Cu2O-dod。实验结果结合密度泛函理论表明,虽然低双配位 O 原子导致 Cu2O-cub 表面氧空位形成能最低 (2.47 eV) 和最高表面 OL 活性,但它无法确定活性。Cu2O-oct 中最低的体氧空位形成能 (3.16 eV) 以三配位 O 原子和开放表面终止,可以加速 OL 的迁移和补充,从而促进催化活性。
更新日期:2024-12-04
中文翻译:

原子配位对晶格氧活性和甲苯对常规 Cu2O 晶体催化氧化的影响
通常通过涉及晶格氧 (OL) 活性的关键作用的 Mars-van Krevelen 机制来理解过渡金属催化剂上的 VOCs 氧化,但是,它如何受到原子配位的影响仍不清楚。在此,我们使用晶面分别为 (100)、(111) 和 (110) 的 Cu2O-cub、Cu2O-oct 和 Cu2O-dod 模型催化剂来研究甲苯的 OL 活性和催化氧化。发现 Cu2O-oct 的活性最高,其次是 Cu2O-cub 和 Cu2O-dod。实验结果结合密度泛函理论表明,虽然低双配位 O 原子导致 Cu2O-cub 表面氧空位形成能最低 (2.47 eV) 和最高表面 OL 活性,但它无法确定活性。Cu2O-oct 中最低的体氧空位形成能 (3.16 eV) 以三配位 O 原子和开放表面终止,可以加速 OL 的迁移和补充,从而促进催化活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号