当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific capture of magnesium ions by phosphorus atomic sites on self-floating nuclei advances Mg/Li separation in salt lakes brine
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136774 Yanyan An, Tuo Wang, Taoran Wang, Wenjuan Yang, Ruqiang Dou, Yatong Jing, Chao Bai, Gu Xu
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136774 Yanyan An, Tuo Wang, Taoran Wang, Wenjuan Yang, Ruqiang Dou, Yatong Jing, Chao Bai, Gu Xu
|
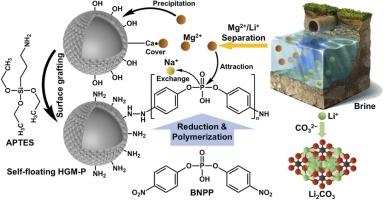
Efficient magnesium-lithium separation is a critical step in extracting lithium ions from salt lakes brine. Precipitation of Mg2+ from liquid to solid is the simplest separation method, but a side reaction of Li+ adsorption by precipitated floc leads to incomplete Mg/Li separation and lithium loss. In this study, we grafted phosphorus atomic sites onto silica-based nuclei with self-floating separation capability to prepare adsorbents with specific capture ability for Mg2+ , achieving efficient Mg/Li separation from brine. Surface composition analysis shows that the content of P element is 0.99 %, which contributed 82.20 mg g⁻1 of Mg2+ adsorption capacity of at room temperature. During this process, the content of Na and Ca elements in the material decreased by 1.47 % and 0.85 %, respectively, due to ion exchange and surface coverage. In samples of the same water quality as Smackover brine, the Li content in the Mg2+ -captured material was only 3.20 % of that in directly precipitated Mg(OH)2 , due to the adsorption selectivity coefficient of the material for Mg²⁺ to Li⁺ reaching 62.26. The outcomes of this research enlighten the selective capture ability and mechanism of phosphorus atomic sites for Mg2+ , providing new insights for efficient Mg/Li separation from salt lakes to improve raw brain grade.
中文翻译:

自浮核上的磷原子位点对镁离子的特异性捕获促进了盐湖盐水中 Mg/Li 的分离
高效的镁-锂分离是从盐湖盐水中提取锂离子的关键步骤。Mg2+ 从液体沉淀到固体是最简单的分离方法,但析出絮凝物吸附 Li+ 的副反应导致 Mg/Li 分离不完全和锂损失。在这项研究中,我们将磷原子位点接枝到具有自浮分离能力的二氧化硅基原子核上,以制备对 Mg2+ 具有特异性捕获能力的吸附剂,实现从盐水中高效分离 Mg/Li。表面成分分析表明,P 元素含量为 0.99 %,在室温下贡献了 82.20 mg g⁻1 的 Mg2+ 吸附容量。在此过程中,由于离子交换和表面覆盖,材料中 Na 和 Ca 元素的含量分别降低了 1.47 % 和 0.85 %。在与 Smackover 盐水水质相同的样品中,由于 Mg²⁺ 对 Li⁺ 的吸附选择性系数达到 62.26,因此 Mg2+ 捕获材料中的 Li 含量仅为直接沉淀 Mg(OH)2 中的 3.20%。本研究结果揭示了磷原子位点对 Mg2+ 的选择性捕获能力和机制,为从盐湖中高效分离 Mg/Li 以提高生脑等级提供了新的见解。
更新日期:2024-12-04
中文翻译:

自浮核上的磷原子位点对镁离子的特异性捕获促进了盐湖盐水中 Mg/Li 的分离
高效的镁-锂分离是从盐湖盐水中提取锂离子的关键步骤。Mg2+ 从液体沉淀到固体是最简单的分离方法,但析出絮凝物吸附 Li+ 的副反应导致 Mg/Li 分离不完全和锂损失。在这项研究中,我们将磷原子位点接枝到具有自浮分离能力的二氧化硅基原子核上,以制备对 Mg2+ 具有特异性捕获能力的吸附剂,实现从盐水中高效分离 Mg/Li。表面成分分析表明,P 元素含量为 0.99 %,在室温下贡献了 82.20 mg g⁻1 的 Mg2+ 吸附容量。在此过程中,由于离子交换和表面覆盖,材料中 Na 和 Ca 元素的含量分别降低了 1.47 % 和 0.85 %。在与 Smackover 盐水水质相同的样品中,由于 Mg²⁺ 对 Li⁺ 的吸附选择性系数达到 62.26,因此 Mg2+ 捕获材料中的 Li 含量仅为直接沉淀 Mg(OH)2 中的 3.20%。本研究结果揭示了磷原子位点对 Mg2+ 的选择性捕获能力和机制,为从盐湖中高效分离 Mg/Li 以提高生脑等级提供了新的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号