当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation of molybdenum disulfide through cascade reactions with hydrogen peroxide in aqueous system
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136794 Dongfang Xu, Jinhao Yao, Yuantong Chi, Zhuomiao Liu, Ruyi Lan, Meng Wang, Wenli Su, Xia Liu, Yanhui Dai, Tongtao Yue, Jian Zhao
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136794 Dongfang Xu, Jinhao Yao, Yuantong Chi, Zhuomiao Liu, Ruyi Lan, Meng Wang, Wenli Su, Xia Liu, Yanhui Dai, Tongtao Yue, Jian Zhao
|
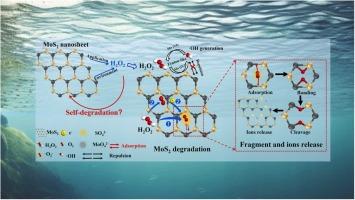
Transformation is a crucial process determining the lifespan and risk of MoS2 nanomaterial during usage and after disposal. This study revealed the degradation of MoS2 in the presence of H2 O2 using experimental and computational methods. Experimental results showed that MoS2 nanosheets were degraded by 45.1 % after 72-h incubation with H2 O2 . MoS2 decomposed H2 O2 into various reactive oxygen species, among which ·O2 - played a dominant role breaking MoS2 into fragments with defects and holes. Mo (IV) in MoS2 catalyzed ·O2 - formation through electron transfer towards H2 O2 . Additionally, electrons generated from cleavage of O-O in H2 O2 initiated the O2 reduction to generate ·O2 - . The interaction of MoS2 with ·O2 - yielded soluble MoO4 2– and SO4 2– , and 22.4 % of Mo on residual MoS2 was in the form of Mo (VI) after 72-h incubation. Density function theory calculations elucidated that·O2 - is more potent than ·OH in adsorbing on MoS2 ( −2.25 eV vs. −0.14 eV) to initiate reaction. The reaction occurred preferentially from Mo and adjacent S atoms, which transferred 1.07 electrons toward ·O2 - to induce O-O cleavage and formation of O-M and O-S bonds. The obtained finding on MoS2 degradation is fundamental for promoting sustainable applications and risk assessment of MoS2 -based nanomaterials.
中文翻译:

通过与过氧化氢在水性体系中的级联反应降解二硫化钼
转化是决定 MoS2 纳米材料在使用期间和处置后的使用寿命和风险的关键过程。本研究使用实验和计算方法揭示了 MoS2 在 H 2 O 2 存在下的降解。实验结果表明,MoS2 纳米片与 H2O2 孵育 72 小时后降解了 45.1 %。MoS2 将 H2O2 分解成各种活性氧,其中 ·O2- 在将 MoS2 分解成具有缺陷和空穴的片段中起主导作用。MoS2 中的 Mo (IV) 催化 ·通过电子向 H2O2 转移形成 O2。此外,H2O2 中 O-O 裂解产生的电子引发了 O2 还原,从而产生 ·O2-。MoS2 与 ·O2- 产生可溶性 MoO42– 和 SO42–,孵育 72 小时后残留 MoS2 上 22.4% 的 Mo 以 Mo (VI) 的形式存在。密度函数理论计算阐明了·O2- 比 ·OH 吸附在 MoS2 上(-2.25 eV vs. -0.14 eV)引发反应。反应优先发生在 Mo 和相邻的 S 原子上,它们向 ·O2- 诱导 O-O 裂解和 O-M 和 O-S 键的形成。获得的 MoS2 降解结果对于促进 MoS2 基纳米材料的可持续应用和风险评估至关重要。
更新日期:2024-12-04
中文翻译:

通过与过氧化氢在水性体系中的级联反应降解二硫化钼
转化是决定 MoS2 纳米材料在使用期间和处置后的使用寿命和风险的关键过程。本研究使用实验和计算方法揭示了 MoS2 在 H 2 O 2 存在下的降解。实验结果表明,MoS2 纳米片与 H2O2 孵育 72 小时后降解了 45.1 %。MoS2 将 H2O2 分解成各种活性氧,其中 ·O2- 在将 MoS2 分解成具有缺陷和空穴的片段中起主导作用。MoS2 中的 Mo (IV) 催化 ·通过电子向 H2O2 转移形成 O2。此外,H2O2 中 O-O 裂解产生的电子引发了 O2 还原,从而产生 ·O2-。MoS2 与 ·O2- 产生可溶性 MoO42– 和 SO42–,孵育 72 小时后残留 MoS2 上 22.4% 的 Mo 以 Mo (VI) 的形式存在。密度函数理论计算阐明了·O2- 比 ·OH 吸附在 MoS2 上(-2.25 eV vs. -0.14 eV)引发反应。反应优先发生在 Mo 和相邻的 S 原子上,它们向 ·O2- 诱导 O-O 裂解和 O-M 和 O-S 键的形成。获得的 MoS2 降解结果对于促进 MoS2 基纳米材料的可持续应用和风险评估至关重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号