当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promoting effect of oxygen vacancies in CuZnOx-2/peroxymonosulfate system on the p-arsanilic acid degradation and secondary arsenic species immobilization
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136742 Chaonan Tao, Kun Wu, Ting Liu, Shengjiong Yang, Zhihua Li
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-04 , DOI: 10.1016/j.jhazmat.2024.136742 Chaonan Tao, Kun Wu, Ting Liu, Shengjiong Yang, Zhihua Li
|
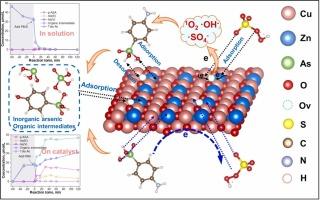
Combining chemical oxidation and adsorption is highly desirable but challenging to remove organoarsenic compounds for water purification. Herein, we prepared a Zn-doped CuO (CuZnOx -2) catalyst by incorporating Zn atoms into the CuO lattice, which results in abundant surface oxygen vacancies (OVs) and modulates the electronic structure of Cu-OVs-Zn sites for PMS activation to degrade p-arsanilic acid (p-ASA) and adsorb the secondary arsenic species simultaneously. The elevated d-band centers for Cu upward to the Fermi level can significantly strengthen the adsorption of PMS, p-ASA, and the generated arsenic species. The OVs cause the charge redistribution to form electron-rich centers, which accelerate the electron transfer from Cu-OVs-Zn sites to adsorbed PMS, facilitating the cleavage of peroxide bond to produce SO4 •− , •OH. Furthermore, the PMS adsorbed on the local environment of OVs with different configurations can directly decompose to produce 1 O2 without undergoing PMS → O2 •− → 1 O2 or O2 → O2 •− → 1 O2 processes. The evolution process of the main arsenic species in solution and catalyst surface with oxidation was clarified. The ultimate removal of the total As involves 20 % As(III), 60 % As(V), and 20 % organic arsenic intermediates via forming inner-sphere complexes or electrostatic interaction. This contribution provides a brand-new perspective for the remediation of organoarsenic pollution over designing highly active catalysts.
中文翻译:

促进CuZnOx-2/过氧一硫酸盐体系中氧空位对砷酸降解和次生砷物种固定化的影响
化学氧化和吸附相结合是非常可取的,但去除有机砷化合物以净化水具有挑战性。在此,我们通过将 Zn 原子掺入 CuO 晶格中制备了一种 Zn 掺杂 CuO (CuZnOx-2) 催化剂,这导致丰富的表面氧空位 (OVs) 并调节 Cu-OVs-Zn 位点的电子结构以激活 PMS,以降解对砷酸 (p-ASA) 并同时吸附仲砷物种。Cu 的 d 带中心升高至费米能级可以显着加强对 PMS、p-ASA 和生成的砷种类的吸附。OV 导致电荷重新分布形成富电子中心,从而加速电子从 Cu-OVs-Zn 位点转移到吸附的 PMS,促进过氧化物键的裂解以产生 SO4•−, •OH。此外,吸附在不同构型的 OVs 局部环境中的 PMS 可以直接分解产生 1O2,而无需→ O2•− → 1O2 或 O2 → O2•− → 1O2 过程。阐明了主要砷种在溶液和催化剂表面的氧化演化过程。总砷的最终去除涉及 20% 的 As(III)、60% 的 As(V) 和 20% 的有机砷中间体,通过形成内球络合物或静电相互作用。这一贡献为有机砷污染的修复提供了全新的视角,而不是设计高活性催化剂。
更新日期:2024-12-04
中文翻译:

促进CuZnOx-2/过氧一硫酸盐体系中氧空位对砷酸降解和次生砷物种固定化的影响
化学氧化和吸附相结合是非常可取的,但去除有机砷化合物以净化水具有挑战性。在此,我们通过将 Zn 原子掺入 CuO 晶格中制备了一种 Zn 掺杂 CuO (CuZnOx-2) 催化剂,这导致丰富的表面氧空位 (OVs) 并调节 Cu-OVs-Zn 位点的电子结构以激活 PMS,以降解对砷酸 (p-ASA) 并同时吸附仲砷物种。Cu 的 d 带中心升高至费米能级可以显着加强对 PMS、p-ASA 和生成的砷种类的吸附。OV 导致电荷重新分布形成富电子中心,从而加速电子从 Cu-OVs-Zn 位点转移到吸附的 PMS,促进过氧化物键的裂解以产生 SO4•−, •OH。此外,吸附在不同构型的 OVs 局部环境中的 PMS 可以直接分解产生 1O2,而无需→ O2•− → 1O2 或 O2 → O2•− → 1O2 过程。阐明了主要砷种在溶液和催化剂表面的氧化演化过程。总砷的最终去除涉及 20% 的 As(III)、60% 的 As(V) 和 20% 的有机砷中间体,通过形成内球络合物或静电相互作用。这一贡献为有机砷污染的修复提供了全新的视角,而不是设计高活性催化剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号