当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photomediated controllable alkylation/nitrosoalkylation of N-heteroaromatics via nucleohomolytic substitution of alkylboronic esters with N-nitrosamines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-04 , DOI: 10.1039/d4qo02008a Ji-Wei Sang, Yu Zhang, Dingding Xia, Zhimin Hu, Jinxin Wang, Wei-Dong Zhang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-04 , DOI: 10.1039/d4qo02008a Ji-Wei Sang, Yu Zhang, Dingding Xia, Zhimin Hu, Jinxin Wang, Wei-Dong Zhang
|
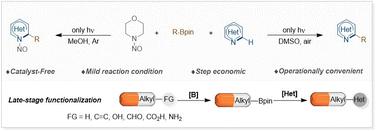
The development of sustainable late-stage functionalization (LSF) methods has become a focal point of research in the field of organic synthesis. Boronic acids and their derivatives are some of the most useful reagents and are suitable triggers for LSF. This study reports the production of diverse alkylated/nitrosoalkylated N-heteroaromatics via the photomediated nucleohomolytic substitution of alkylboronic esters with N-nitrosamines in the absence of a photocatalyst. This green approach facilitates LSF through various protodeboronation sequences for natural products and drug molecules, expanding the biologically relevant chemical space.
中文翻译:

通过烷基硼酯的核同溶取代 N-亚硝胺,光介导的可控烷基化/亚硝基烷基化
可持续的后期功能化 (LSF) 方法的开发已成为有机合成领域研究的重点。硼酸及其衍生物是一些最有用的试剂,是 LSF 的合适触发器。本研究报道了在没有光催化剂的情况下,通过光介导的烷基硼酯用 N-亚硝基胺的光介导核均解取代烷基硼酯产生多种烷基化/亚硝基烷基化 N-杂芳烃。这种绿色方法通过天然产物和药物分子的各种原脱硼序列促进 LSF,从而扩展了生物学相关的化学空间。
更新日期:2024-12-09
中文翻译:

通过烷基硼酯的核同溶取代 N-亚硝胺,光介导的可控烷基化/亚硝基烷基化
可持续的后期功能化 (LSF) 方法的开发已成为有机合成领域研究的重点。硼酸及其衍生物是一些最有用的试剂,是 LSF 的合适触发器。本研究报道了在没有光催化剂的情况下,通过光介导的烷基硼酯用 N-亚硝基胺的光介导核均解取代烷基硼酯产生多种烷基化/亚硝基烷基化 N-杂芳烃。这种绿色方法通过天然产物和药物分子的各种原脱硼序列促进 LSF,从而扩展了生物学相关的化学空间。






























 京公网安备 11010802027423号
京公网安备 11010802027423号