当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-activity relationship study of novel evodiamine amino acid conjugates with potent anti-colorectal cancer efficacy
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-03 , DOI: 10.1016/j.ejmech.2024.117132 Shuting Chen, Xi Zhang, Hanxuan Mo, Ying Peng, Zhigang An, Junbo Wu, Xiuzhen Wei, Siyi Zhang, Yongxia Xiong, Weifan Jiang, Xue Peng, Linsheng Zhuo, Zhengwen Lei, Zhen Wang, Zecheng Hu
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-12-03 , DOI: 10.1016/j.ejmech.2024.117132 Shuting Chen, Xi Zhang, Hanxuan Mo, Ying Peng, Zhigang An, Junbo Wu, Xiuzhen Wei, Siyi Zhang, Yongxia Xiong, Weifan Jiang, Xue Peng, Linsheng Zhuo, Zhengwen Lei, Zhen Wang, Zecheng Hu
|
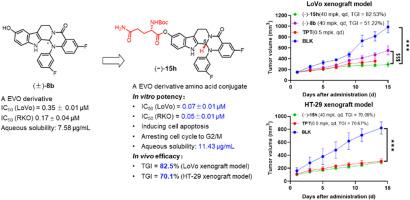
Evodiamine has been a promising lead structure with broad-spectrum antitumor activity. Druggability optimization is the most challenging part of evodiamine-based lead-to-candidate campaign. Amino acids as building blocks for conjugates are widely incorporated into approved drug and drug candidates, demonstrating highly attractive druggability. Herein, a series of evodiamine amino acid conjugates were designed and synthesized based on the evodiamine lead compound (±)-8b discovered in our previous work. The structure−activity relationship (SAR) studies culminated in the identification of a promising conjugate (−)-15h featuring a N -Boc-l sinister ), which exhibited nanomolar antiproliferative activity against LoVo and RKO colorectal cancer cells. Moreover, (−)-15h could inhibit topoisomerases I, arrest the cell cycle in the G2/M phase, and induce apoptosis. Importantly, (−)-15h (tumor growth inhibition rate was 82.53 % in 40 mpk) showed better efficacy and tolerability to that of parent compound (−)-8b (tumor growth inhibition rate was 51.22 % in 40 mpk) in LoVo xenograft model. Further, (−)-15h (tumor growth inhibition rate was 70.09 % in 40 mpk) showed comparable efficacy and better tolerability to that of topotecan (tumor growth inhibition rate was 70.67 % in 0.5 mpk) in HT-29 xenograft model. Collectively, this study further provided a strong scientific basis for amino acid-based structural modifications and a drug lead for anti-colorectal cancer applications.
中文翻译:

具有强效抗结直肠癌疗效的新型吴茱萸碱氨基酸偶联物的构效关系研究
吴茱萸碱是一种很有前途的先导结构,具有广谱抗肿瘤活性。成药性优化是基于吴茱萸碱的潜在客户到候选人活动中最具挑战性的部分。氨基酸作为偶联物的组成部分被广泛纳入已批准的药物和候选药物中,显示出极具吸引力的成药性。在此,基于我们之前工作中发现的吴茱萸碱先导化合物 (±)-8b 设计和合成了一系列吴茱萸碱氨基酸偶联物。构效关系 (SAR) 研究最终鉴定出一种有前途的偶联物 (-)-15h,该偶联物具有 N-Boc-l-谷氨酰胺基团和手性碳原子 (sinister),其对 LoVo 和 RKO 结直肠癌细胞表现出纳摩尔抗增殖活性。此外,(-)-15h 可以抑制拓扑异构酶 I,将细胞周期停在 G2/M 期,并诱导细胞凋亡。重要的是,(-)-15h (40 mpk 中肿瘤生长抑制率为 82.53 %)在 LoVo 异种移植模型中显示出优于母体化合物 (-)-8b (40 mpk 中肿瘤生长抑制率为 51.22 %)的疗效和耐受性。此外,(-)-15h (40 mpk 中肿瘤生长抑制率为 70.09 %)在 HT-29 异种移植模型中显示出与拓扑替康相当的疗效和更好的耐受性 (0.5 mpk 中肿瘤生长抑制率为 70.67 %)。总的来说,这项研究进一步为基于氨基酸的结构修饰和抗结直肠癌应用的药物线索提供了强有力的科学基础。
更新日期:2024-12-03
中文翻译:

具有强效抗结直肠癌疗效的新型吴茱萸碱氨基酸偶联物的构效关系研究
吴茱萸碱是一种很有前途的先导结构,具有广谱抗肿瘤活性。成药性优化是基于吴茱萸碱的潜在客户到候选人活动中最具挑战性的部分。氨基酸作为偶联物的组成部分被广泛纳入已批准的药物和候选药物中,显示出极具吸引力的成药性。在此,基于我们之前工作中发现的吴茱萸碱先导化合物 (±)-8b 设计和合成了一系列吴茱萸碱氨基酸偶联物。构效关系 (SAR) 研究最终鉴定出一种有前途的偶联物 (-)-15h,该偶联物具有 N-Boc-l-谷氨酰胺基团和手性碳原子 (sinister),其对 LoVo 和 RKO 结直肠癌细胞表现出纳摩尔抗增殖活性。此外,(-)-15h 可以抑制拓扑异构酶 I,将细胞周期停在 G2/M 期,并诱导细胞凋亡。重要的是,(-)-15h (40 mpk 中肿瘤生长抑制率为 82.53 %)在 LoVo 异种移植模型中显示出优于母体化合物 (-)-8b (40 mpk 中肿瘤生长抑制率为 51.22 %)的疗效和耐受性。此外,(-)-15h (40 mpk 中肿瘤生长抑制率为 70.09 %)在 HT-29 异种移植模型中显示出与拓扑替康相当的疗效和更好的耐受性 (0.5 mpk 中肿瘤生长抑制率为 70.67 %)。总的来说,这项研究进一步为基于氨基酸的结构修饰和抗结直肠癌应用的药物线索提供了强有力的科学基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号