当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ClpS Directs Degradation of N‐Degron Substrates With Primary Destabilizing Residues in Mycolicibacterium smegmatis
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-12-03 , DOI: 10.1111/mmi.15334 Christopher J. Presloid, Jialiu Jiang, Pratistha Kandel, Henry R. Anderson, Patrick C. Beardslee, Thomas M. Swayne, Karl R. Schmitz
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-12-03 , DOI: 10.1111/mmi.15334 Christopher J. Presloid, Jialiu Jiang, Pratistha Kandel, Henry R. Anderson, Patrick C. Beardslee, Thomas M. Swayne, Karl R. Schmitz
|
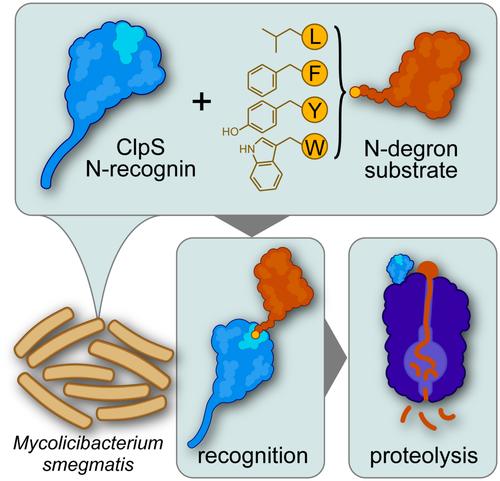
Drug‐resistant tuberculosis infections are a major threat to global public health. The essential mycobacterial ClpC1P1P2 protease has received attention as a prospective target for novel antibacterial therapeutics. However, efforts to probe its function in cells are constrained by our limited knowledge of its physiological proteolytic repertoire. Here, we interrogate the role of mycobacterial ClpS in directing N‐degron pathway proteolysis by ClpC1P1P2 in Mycolicibacterium smegmatis . Binding assays demonstrate that mycobacterial ClpS binds canonical primary destabilizing residues (Leu, Phe, Tyr, Trp) with moderate affinity. N‐degron binding restricts the conformational flexibility of a loop adjacent to the ClpS N‐degron binding pocket and strengthens ClpS•ClpC1 binding affinity ~30‐fold, providing a mechanism for cells to prioritize N‐degron proteolysis when substrates are abundant. Proteolytic reporter assays in M. smegmatis confirm degradation of substrates bearing primary N‐degrons, but suggest that secondary N‐degrons are absent in mycobacteria. This work expands our understanding of the mycobacterial N‐degron pathway and identifies ClpS as a critical component for substrate specificity, providing insights that may support the development of improved Clp protease inhibitors.
中文翻译:

ClpS 指导耻垢分枝杆菌中具有原发性不稳定残基的 N-Degron 底物的降解
耐药结核病感染是全球公共卫生的主要威胁。必需的分枝杆菌 ClpC1P1P2 蛋白酶作为新型抗菌疗法的前瞻性靶点而受到关注。然而,由于我们对它的生理蛋白水解库的了解有限,探测其在细胞中的功能的努力受到限制。在这里,我们询问了分枝杆菌 ClpS 在耻垢分枝杆菌中通过 ClpC1P1P2 指导 N-degron 途径蛋白水解中的作用。结合测定表明,分枝杆菌 ClpS 以中等亲和力结合经典的原代不稳定残基 (Leu、Phe、Tyr、Trp)。N-degron 结合限制了与 ClpS N-degron 结合口袋相邻的环的构象灵活性,并增强了 ClpS•ClpC1 结合亲和力 ~30 倍,为细胞提供了一种机制,当底物丰富时,细胞可以优先考虑 N-degron 蛋白水解。M. smegmatis 中的蛋白水解报告基因测定证实了带有初级 N-degrons 的底物的降解,但表明分枝杆菌中不存在次级 N-degrons。这项工作扩展了我们对分枝杆菌 N-degron 通路的理解,并确定 ClpS 是底物特异性的关键组分,为可能支持开发改进的 Clp 蛋白酶抑制剂提供了见解。
更新日期:2024-12-03
中文翻译:

ClpS 指导耻垢分枝杆菌中具有原发性不稳定残基的 N-Degron 底物的降解
耐药结核病感染是全球公共卫生的主要威胁。必需的分枝杆菌 ClpC1P1P2 蛋白酶作为新型抗菌疗法的前瞻性靶点而受到关注。然而,由于我们对它的生理蛋白水解库的了解有限,探测其在细胞中的功能的努力受到限制。在这里,我们询问了分枝杆菌 ClpS 在耻垢分枝杆菌中通过 ClpC1P1P2 指导 N-degron 途径蛋白水解中的作用。结合测定表明,分枝杆菌 ClpS 以中等亲和力结合经典的原代不稳定残基 (Leu、Phe、Tyr、Trp)。N-degron 结合限制了与 ClpS N-degron 结合口袋相邻的环的构象灵活性,并增强了 ClpS•ClpC1 结合亲和力 ~30 倍,为细胞提供了一种机制,当底物丰富时,细胞可以优先考虑 N-degron 蛋白水解。M. smegmatis 中的蛋白水解报告基因测定证实了带有初级 N-degrons 的底物的降解,但表明分枝杆菌中不存在次级 N-degrons。这项工作扩展了我们对分枝杆菌 N-degron 通路的理解,并确定 ClpS 是底物特异性的关键组分,为可能支持开发改进的 Clp 蛋白酶抑制剂提供了见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号