当前位置:
X-MOL 学术
›
Ann. N. Y. Acad. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and safety of the glucagon receptor antagonist volagidemab in type‐1 diabetes: A systematic review and meta‐analysis
Annals of the New York Academy of Sciences ( IF 4.1 ) Pub Date : 2024-12-03 , DOI: 10.1111/nyas.15262 Deep Dutta, A. B. M. Kamrul‐Hasan, Vineet Surana, Rajiv Singla, Deepak Khandelwal, Sameer Aggarwal, Lakshmi Nagendra, Saptarshi Bhattacharya
Annals of the New York Academy of Sciences ( IF 4.1 ) Pub Date : 2024-12-03 , DOI: 10.1111/nyas.15262 Deep Dutta, A. B. M. Kamrul‐Hasan, Vineet Surana, Rajiv Singla, Deepak Khandelwal, Sameer Aggarwal, Lakshmi Nagendra, Saptarshi Bhattacharya
|
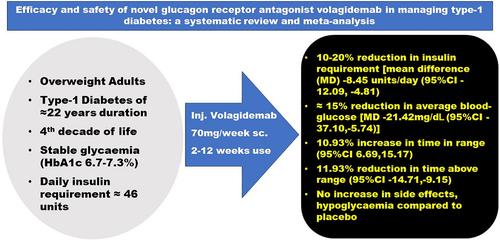
The glucagon receptor antagonist (GRA) volagidemab is the first‐in‐class fully human monoclonal antibody that inhibits glucagon receptor. GRA can improve glycemia by reducing endogenous glucose production and reduce risks of diabetic ketoacidosis by suppressing ketogenesis. This systematic review and meta‐analysis analyzed the efficacy and safety of volagidemab in type‐1 diabetes (T1D). Electronic databases were searched for randomized controlled trials (RCTs) involving T1D patients receiving volagidemab. The primary outcome was to evaluate changes in total daily dose (TDD) of insulin. The secondary outcomes were to evaluate changes in measures of glycemia, hypoglycemia, and adverse events. Data from 3 RCTs (98 patients) were analyzed. Volagidemab (70 mg/week) was associated with a significant reduction in TDD of insulin requirement (mean difference [MD]: −8.45 units/day (95% confidence interval [CI]: [−12.09, −4.81]); I 2 = 83%; p < 0.01) and average blood glucose (MD: −21.42 mg/dL (95% CI: [−37.10, −5.74]); I 2 = 88%; p < 0.01), compared to placebo. Volagidemab use was associated with a significant increase in time in range (blood glucose: 70–180 mg/dL) (MD: 10.93% (95% CI: [6.69, 15.17]); I 2 = 55%; p < 0.01) and significant reduction in time above range (blood glucose >180 mg/dL) (MD: −11.93% (95% CI: [−14.71, −9.15]); I 2 = 6%; p < 0.01) without any impact on time below range (blood glucose <70 mg/dL) (MD: 0.14% (95% CI: [−0.56, 0.84]); I 2 = 0%; p = 0.70), compared to placebo. Occurrence of treatment‐emergent adverse events (odds ratio [OR]: 0.96 (95% CI: [0.36, 2.56]); I 2 = 8%; p = 0.94) and hypoglycemia (OR: 0.56 (95% CI: [0.11, 2.89]); I 2 = 0%; p = 0.49) were similar among volagidemab users as compared to placebo. Short‐term volagidemab use was associated with significant reduction in insulin requirement along with improvement in glycemia.
中文翻译:

胰高血糖素受体拮抗剂 volagidemab 治疗 1 型糖尿病的疗效和安全性:系统评价和荟萃分析
胰高血糖素受体拮抗剂 (GRA) volagidemab 是抑制胰高血糖素受体的同类首创全人源单克隆抗体。GRA 可以通过减少内源性葡萄糖的产生来改善血糖,并通过抑制酮症生成来降低糖尿病酮症酸中毒的风险。本系统评价和荟萃分析分析了 volagidemab 在 1 型糖尿病 (T1D) 中的疗效和安全性。在电子数据库中检索涉及接受 volagidemab 的 T1D 患者的随机对照试验 (RCT)。主要结局是评估胰岛素每日总剂量 (TDD) 的变化。次要结局是评估血糖、低血糖和不良事件测量的变化。分析了 3 项 RCT (98 例患者) 的数据。Volagidemab (70 mg/周) 与胰岛素需求 TDD 的显著降低相关 (平均差 [MD]: -8.45 单位/天 (95% 置信区间 [CI]: [-12.09, -4.81]);I2 = 83%;p < 0.01) 和平均血糖 (MD: -21.42 mg/dL (95% CI: [-37.10, -5.74]);I2 = 88%;p < 0.01),与安慰剂相比。Volagidemab 的使用与范围时间的显着增加相关(血糖:70-180 mg/dL)(MD:10.93%(95% CI:[6.69,15.17]);I2 = 55%;p < 0.01) 和超出范围的时间显着减少 (血糖 >180 mg/dL) (MD: -11.93% (95% CI: [-14.71, -9.15]);I2 = 6%;p < 0.01) 对低于范围的时间 (血糖 <70 mg/dL) 没有任何影响 (MD: 0.14% (95% CI: [-0.56, 0.84]);I2 = 0%;p = 0.70)。治疗中出现的不良事件的发生率(比值比 [OR]: 0.96 (95% CI: [0.36, 2.56]);I2 = 8%;p = 0.94) 和低血糖 (OR: 0.56 (95% CI: [0.11, 2.89]);I2 = 0%;p = 0.49)在服用 volagidemab 的用户中与安慰剂组相似。 短期使用 volagidemab 与胰岛素需求量的显着降低以及血糖的改善有关。
更新日期:2024-12-03
中文翻译:

胰高血糖素受体拮抗剂 volagidemab 治疗 1 型糖尿病的疗效和安全性:系统评价和荟萃分析
胰高血糖素受体拮抗剂 (GRA) volagidemab 是抑制胰高血糖素受体的同类首创全人源单克隆抗体。GRA 可以通过减少内源性葡萄糖的产生来改善血糖,并通过抑制酮症生成来降低糖尿病酮症酸中毒的风险。本系统评价和荟萃分析分析了 volagidemab 在 1 型糖尿病 (T1D) 中的疗效和安全性。在电子数据库中检索涉及接受 volagidemab 的 T1D 患者的随机对照试验 (RCT)。主要结局是评估胰岛素每日总剂量 (TDD) 的变化。次要结局是评估血糖、低血糖和不良事件测量的变化。分析了 3 项 RCT (98 例患者) 的数据。Volagidemab (70 mg/周) 与胰岛素需求 TDD 的显著降低相关 (平均差 [MD]: -8.45 单位/天 (95% 置信区间 [CI]: [-12.09, -4.81]);I2 = 83%;p < 0.01) 和平均血糖 (MD: -21.42 mg/dL (95% CI: [-37.10, -5.74]);I2 = 88%;p < 0.01),与安慰剂相比。Volagidemab 的使用与范围时间的显着增加相关(血糖:70-180 mg/dL)(MD:10.93%(95% CI:[6.69,15.17]);I2 = 55%;p < 0.01) 和超出范围的时间显着减少 (血糖 >180 mg/dL) (MD: -11.93% (95% CI: [-14.71, -9.15]);I2 = 6%;p < 0.01) 对低于范围的时间 (血糖 <70 mg/dL) 没有任何影响 (MD: 0.14% (95% CI: [-0.56, 0.84]);I2 = 0%;p = 0.70)。治疗中出现的不良事件的发生率(比值比 [OR]: 0.96 (95% CI: [0.36, 2.56]);I2 = 8%;p = 0.94) 和低血糖 (OR: 0.56 (95% CI: [0.11, 2.89]);I2 = 0%;p = 0.49)在服用 volagidemab 的用户中与安慰剂组相似。 短期使用 volagidemab 与胰岛素需求量的显着降低以及血糖的改善有关。






























 京公网安备 11010802027423号
京公网安备 11010802027423号