Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fast ionic conduction achieved through the design and synthesis of ceramic heterointerfaces
Joule ( IF 38.6 ) Pub Date : 2024-12-03 , DOI: 10.1016/j.joule.2024.11.006 Shingo Ohta, Nikhilendra Singh, Rajeev Kumar Rai, Hyeongjun Koh, Yihui Zhang, Wonjoon Suk, Max J. Palmer, Son-Jong Hwang, Michael Jones, Chuhong Wang, Chen Ling, Kimber Stamm Masias, Eli Stavitski, Jeff Sakamoto, Eric A. Stach
Joule ( IF 38.6 ) Pub Date : 2024-12-03 , DOI: 10.1016/j.joule.2024.11.006 Shingo Ohta, Nikhilendra Singh, Rajeev Kumar Rai, Hyeongjun Koh, Yihui Zhang, Wonjoon Suk, Max J. Palmer, Son-Jong Hwang, Michael Jones, Chuhong Wang, Chen Ling, Kimber Stamm Masias, Eli Stavitski, Jeff Sakamoto, Eric A. Stach
|
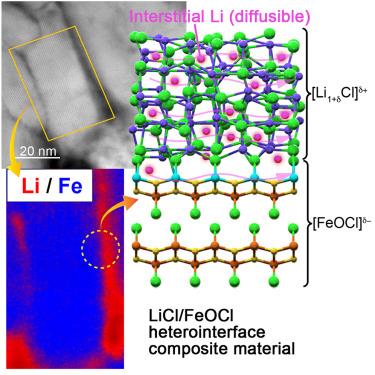
Lithium (Li) chloride and iron oxychloride (FeOCl), typically nonconductive, were combined to form a [Li1+δCl]δ+/[FeOCl]δ− heterointerface composite material (LFH), achieving ionic conductivities of >1 mS cm−1. Analysis techniques (scanning transmission electron microscopy [STEM] and electron energy-loss spectroscopy [EELS]) indicated that the microstructure of LFH consisted of an amorphous LiCl-based shell surrounding a crystalline FeOCl-based core. Electrochemical measurements alongside solid-state 6,7Li nuclear magnetic resonance (NMR) and molecular dynamic simulations revealed Li+ as the sole conductive species, with a diffusion barrier of ∼0.25 eV. X-ray photoelectron spectroscopy (XPS) and X-ray absorption fine structure (XAFS) results further supported interstitial Li+ diffusion at the heterointerface and within the LiCl phase, made possible by the heterointerface. Despite susceptibility to electronic conductivity, iron’s defects and multivalency (Fe³⁺, Fe²⁺) enable the Fe–O–Cl framework to accept Cl−, facilitating Li⁺ ionic conduction. A prototype solid-state cell (showing 97% Coulombic efficiency) demonstrated the viability of this heterointerface design for applications in energy storage.
中文翻译:

通过设计和合成陶瓷异质界面实现快速离子传导
氯化锂 (Li) 和氯铁 (FeOCl),通常是不导电的,结合形成 [Li1+δCl]δ+/[FeOCl]δ− 异质界面复合材料 (LFH),实现 >1 mS cm−1 的离子电导率。分析技术(扫描透射电子显微镜 [STEM] 和电子能量损失光谱 [EELS])表明,LFH 的微观结构由围绕结晶 FeOCl 基核心的非晶态 LiCl 基壳组成。电化学测量以及固态 6,7Li 核磁共振 (NMR) 和分子动力学模拟显示 Li+ 是唯一的导电物质,扩散势垒为 ∼0.25 eV。X 射线光电子能谱 (XPS) 和 X 射线吸收精细结构 (XAFS) 结果进一步支持了异质界面和 LiCl 相内的间隙 Li+ 扩散,这由异质界面实现。尽管对电子导电性敏感,但铁的缺陷和多价性(Fe³⁺、Fe²⁺)使 Fe-O-Cl 框架能够接受 Cl−,从而促进 Li⁺ 离子传导。原型固态电池(显示 97% 的库仑效率)证明了这种异质界面设计在储能应用中的可行性。
更新日期:2024-12-03
中文翻译:

通过设计和合成陶瓷异质界面实现快速离子传导
氯化锂 (Li) 和氯铁 (FeOCl),通常是不导电的,结合形成 [Li1+δCl]δ+/[FeOCl]δ− 异质界面复合材料 (LFH),实现 >1 mS cm−1 的离子电导率。分析技术(扫描透射电子显微镜 [STEM] 和电子能量损失光谱 [EELS])表明,LFH 的微观结构由围绕结晶 FeOCl 基核心的非晶态 LiCl 基壳组成。电化学测量以及固态 6,7Li 核磁共振 (NMR) 和分子动力学模拟显示 Li+ 是唯一的导电物质,扩散势垒为 ∼0.25 eV。X 射线光电子能谱 (XPS) 和 X 射线吸收精细结构 (XAFS) 结果进一步支持了异质界面和 LiCl 相内的间隙 Li+ 扩散,这由异质界面实现。尽管对电子导电性敏感,但铁的缺陷和多价性(Fe³⁺、Fe²⁺)使 Fe-O-Cl 框架能够接受 Cl−,从而促进 Li⁺ 离子传导。原型固态电池(显示 97% 的库仑效率)证明了这种异质界面设计在储能应用中的可行性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号