当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biogeochemical interaction between thallium (Tl) and schwertmannite in acidic environment and the anti-dissolution mechanisms of Tl(I)-coprecipitated schwertmannite
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-03 , DOI: 10.1016/j.jhazmat.2024.136764 Liangjing Zhang, Liping Zhang, Liuwei Wang, Deyi Hou
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-03 , DOI: 10.1016/j.jhazmat.2024.136764 Liangjing Zhang, Liping Zhang, Liuwei Wang, Deyi Hou
|
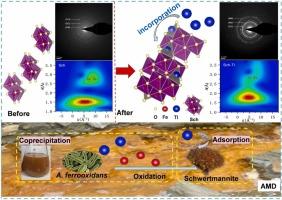
Highly toxic thallium (Tl) can be released into the environment through acid mine drainage (AMD). However, our knowledge on the biogeochemical processes of Tl in such acidic, iron (Fe)-rich environments is limited. Here, we show that schwertmannite, a naturally formed Fe(III) mineral in AMD, can effectively immobilize Tl(I) through coprecipitation and adsorption. Tl(I) coprecipitation into schwertmannite removed a large portion of Tl(I) under a wide range of initial Tl(I) concentrations (0.01–1.0 mg/L) and within a short duration (48 h). The saturated adsorption capacities of the biosynthetic and chemically synthesized schwertmannite for Tl(I) (1.0 mg/L) were 1.96 and 1.59 mg/g, respectively, under acidic conditions (pH=3.0). The kinetic dissolution results indicated that biogenic Tl-coprecipitated schwertmannite exhibited greater stability, which was attributed mainly to the elevated extent of Tl oxidation and enhanced crystallinity of Tl-bearing schwertmannite. The extended X-ray absorption fine structure (EXAFS) analyses revealed that the incorporation of Tl into schwertmannite involves the heterovalent substitution of Fe(III) by the formation of double-corner sharing linkages between the Tl-O tetrahedra and Fe-O octahedra. These results suggested that coprecipitation combined with adsorption can achieve retention of Tl in acidic environment throughout the entire mineralization process of schwertmannite, which provides a comprehensive understanding of biogeochemical fate of Tl in AMD-affected areas.
中文翻译:

酸性环境中铊 (Tl) 与瑞士wertmannite 的生物地球化学相互作用及 Tl(I) 共沉淀 schwertmannite 的抗溶出机制
剧毒铊 (Tl) 可通过酸性矿山排水 (AMD) 释放到环境中。然而,我们对这种酸性、富含铁 (Fe) 的环境中 Tl 的生物地球化学过程的了解是有限的。在这里,我们展示了 schwertmannite 是 AMD 中天然形成的 Fe(III) 矿物,可以通过共沉淀和吸附有效地固定 Tl(I)。Tl(I) 共沉淀到瑞士wertmannite 中,在较宽的初始 Tl(I) 浓度范围 (0.01–1.0 mg/L) 和短持续时间 (48 h) 内去除了大部分 Tl(I)。在酸性条件下 (pH=3.0),生物合成和化学合成的 schwertmannite 对 Tl(I) (1.0 mg/L) 的饱和吸附能力分别为 1.96 和 1.59 mg/g。动力学溶解结果表明,生物成因 Tl-共沉淀的 schwertmannite 表现出更高的稳定性,这主要归因于 Tl 氧化程度的增加和含 Tl 的 schwertmannite 结晶度的增强。扩展 X 射线吸收精细结构 (EXAFS) 分析表明,Tl 掺入 schwertmannite 中涉及 Fe(III) 的异价取代,通过在 Tl-O 四面体和 Fe-O 八面体之间形成双角共享键。这些结果表明,共沉淀结合吸附可以实现 Tl 在酸性环境中的保留,贯穿 schwertmannite 的整个矿化过程,这为全面了解 Tl 在 AMD 影响地区的生物地球化学归宿提供了新的信息。
更新日期:2024-12-03
中文翻译:

酸性环境中铊 (Tl) 与瑞士wertmannite 的生物地球化学相互作用及 Tl(I) 共沉淀 schwertmannite 的抗溶出机制
剧毒铊 (Tl) 可通过酸性矿山排水 (AMD) 释放到环境中。然而,我们对这种酸性、富含铁 (Fe) 的环境中 Tl 的生物地球化学过程的了解是有限的。在这里,我们展示了 schwertmannite 是 AMD 中天然形成的 Fe(III) 矿物,可以通过共沉淀和吸附有效地固定 Tl(I)。Tl(I) 共沉淀到瑞士wertmannite 中,在较宽的初始 Tl(I) 浓度范围 (0.01–1.0 mg/L) 和短持续时间 (48 h) 内去除了大部分 Tl(I)。在酸性条件下 (pH=3.0),生物合成和化学合成的 schwertmannite 对 Tl(I) (1.0 mg/L) 的饱和吸附能力分别为 1.96 和 1.59 mg/g。动力学溶解结果表明,生物成因 Tl-共沉淀的 schwertmannite 表现出更高的稳定性,这主要归因于 Tl 氧化程度的增加和含 Tl 的 schwertmannite 结晶度的增强。扩展 X 射线吸收精细结构 (EXAFS) 分析表明,Tl 掺入 schwertmannite 中涉及 Fe(III) 的异价取代,通过在 Tl-O 四面体和 Fe-O 八面体之间形成双角共享键。这些结果表明,共沉淀结合吸附可以实现 Tl 在酸性环境中的保留,贯穿 schwertmannite 的整个矿化过程,这为全面了解 Tl 在 AMD 影响地区的生物地球化学归宿提供了新的信息。






























 京公网安备 11010802027423号
京公网安备 11010802027423号