当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploration of functional group effects on D2/H2 separation selectivity within the UiO-66 framework
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2024-12-03 , DOI: 10.1039/d4qi02802c Xiufang Li, Yanxi Tan, Zhanfeng Ju, Wenjing Wang, Daqiang Yuan
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2024-12-03 , DOI: 10.1039/d4qi02802c Xiufang Li, Yanxi Tan, Zhanfeng Ju, Wenjing Wang, Daqiang Yuan
|
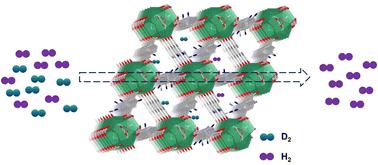
The efficient separation of deuterium from hydrogen remains a significant challenge due to the limitations of conventional techniques, such as cryogenic distillation and the Girdler-sulfide process combined with electrolysis, which are char-acterized by substantial energy demands and relatively low separation coefficients. In contrast, the quantum sieving effect, based on porous materials, offers a promising approach to overcoming these challenges. This study presents a novel application of strong adsorption sites (μ3-OH group) within the nanoporous metal–organic framework of UiO-66 for hydrogen isotope separation. By incorporating diverse organic functional groups into UiO-66, we successfully synthesized four derivative materials: UiO-66–NH2, UiO-66–CH3, UiO-66–NO2, and UiO-66–Ph. Experimental data reveal that the introduction of these functional groups modulated the material's pore size and channel polarity, significantly impacting its adsorption and separation performance for hydrogen isotopes. Notably, UiO-66–NH2, with the smallest pore size and highest channel polarity, exhibited superior hydrogen isotope adsorption capacity and selectivity, highlighting its potential as an effective adsorbent for isotope separation.
中文翻译:

探索 UiO-66 框架内官能团对 D2/H2 分离选择性的影响
由于传统技术(例如低温蒸馏和 Girdler 硫化物工艺与电解相结合)的局限性,氘与氢的有效分离仍然是一项重大挑战,这些技术具有大量的能源需求和相对较低的分离系数的特点。相比之下,基于多孔材料的量子筛分效应为克服这些挑战提供了一种很有前途的方法。本研究提出了 UiO-66 纳米多孔金属有机框架内强吸附位点 (μ3-OH 基团) 用于氢同位素分离的新应用。通过将不同的有机官能团掺入 UiO-66 中,我们成功合成了四种衍生材料:UiO-66-NH2、UiO-66-CH3、UiO-66-NO2 和 UiO-66-Ph。实验数据表明,这些官能团的引入调节了材料的孔径和通道极性,显着影响了其对氢同位素的吸附和分离性能。值得注意的是,UiO-66-NH2 具有最小的孔径和最高的通道极性,表现出优异的氢同位素吸附能力和选择性,突出了其作为有效同位素分离吸附剂的潜力。
更新日期:2024-12-06
中文翻译:

探索 UiO-66 框架内官能团对 D2/H2 分离选择性的影响
由于传统技术(例如低温蒸馏和 Girdler 硫化物工艺与电解相结合)的局限性,氘与氢的有效分离仍然是一项重大挑战,这些技术具有大量的能源需求和相对较低的分离系数的特点。相比之下,基于多孔材料的量子筛分效应为克服这些挑战提供了一种很有前途的方法。本研究提出了 UiO-66 纳米多孔金属有机框架内强吸附位点 (μ3-OH 基团) 用于氢同位素分离的新应用。通过将不同的有机官能团掺入 UiO-66 中,我们成功合成了四种衍生材料:UiO-66-NH2、UiO-66-CH3、UiO-66-NO2 和 UiO-66-Ph。实验数据表明,这些官能团的引入调节了材料的孔径和通道极性,显着影响了其对氢同位素的吸附和分离性能。值得注意的是,UiO-66-NH2 具有最小的孔径和最高的通道极性,表现出优异的氢同位素吸附能力和选择性,突出了其作为有效同位素分离吸附剂的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号