Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-12-02 , DOI: 10.1002/adsc.202401349 Anil Balajirao Dapkekar, Suman Kumar Nag, Gedu Satyanarayana
|
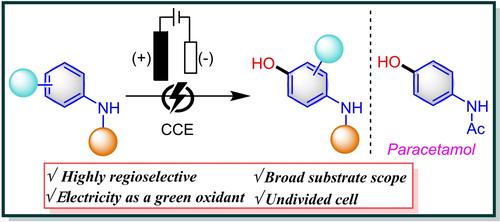
Organic synthesis has long been fascinated by phenolic compounds, especially their exploration of selectively hydroxylating arenes utilizing H2O as a hydroxyl source. However, phenols′ low redox potential and strong reactivity frequently result in unwanted overoxidation byproducts. Here, we present an electrochemical strategy to overcome this difficulty by using electricity as an oxidant and facilitating the para-selective hydroxylation of N-protected anilines. This process exhibits excellent regio-selectivity, compatibility with diverse functional groups, and adaptability by handling an extensive range of substrates. Noteworthily, the technique provides a sustainable substitute for paracetamol synthesis and terminal acetylene-holding hydroxylated products, proving the suitability of this strategy for drug synthesis. Significantly, mechanistic investigations suggest the possible radical pathway and water as a hydroxyl source for this strategy.
中文翻译:

电化学驱动的位点选择性 C(sp2)−H 键羟基化 N-取代苯胺
长期以来,有机合成一直对酚类化合物着迷,尤其是他们利用 H2O 作为羟基源选择性羟基化芳烃的探索。然而,酚类的低氧化还原电位和强反应性经常导致不需要的过度氧化副产物。在这里,我们提出了一种电化学策略,通过使用电作为氧化剂并促进 N 保护苯胺的对位选择性羟基化来克服这一困难。该工艺表现出优异的区域选择性、与不同官能团的相容性,以及处理各种底物的适应性。值得注意的是,该技术为扑热息痛合成和末端持有乙炔的羟基化产物提供了一种可持续的替代品,证明了这种策略适用于药物合成。值得注意的是,机理研究表明可能的自由基途径和水是该策略的羟基来源。






























 京公网安备 11010802027423号
京公网安备 11010802027423号