当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt-catalyzed regio- and diastereoselective carbocyclization/arylation of 1,5-bisallenes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-03 , DOI: 10.1039/d4qo01818d Tao Wang, Hua Huang, Ji-Xun Guan, Xiao-Die An, Yun-Xuan Tan, Ping Tian
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-12-03 , DOI: 10.1039/d4qo01818d Tao Wang, Hua Huang, Ji-Xun Guan, Xiao-Die An, Yun-Xuan Tan, Ping Tian
|
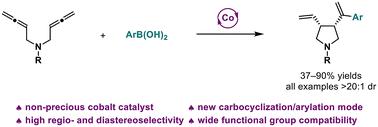
Transition metal-catalyzed carbocyclization of allenes is a versatile method for the construction of structurally diverse cyclic compounds. The carbocyclization of bisallenes is less explored due to the inherent difficulty in controlling the reaction reactivity and selectivity. Herein, we report the first non-precious cobalt-catalyzed carbocyclization/arylation of 1,5-bisallenes with aryl boronic acids, affording cis-disubstituted pyrroles with high regio- and diastereoselectivity, as well as wide functional group compatibility. Moreover, a subgram-scale experiment and downstream transformations are presented.
中文翻译:

钴催化的 1,5-双丙烯的区域和非对映选择性碳环化/芳基化
化丙烯的过渡金属催化碳环化是构建结构多样的环状化合物的通用方法。由于控制反应反应性和选择性的固有困难,双烯烃的碳环化研究较少。在本文中,我们报道了 1,5-双烯与芳基硼酸的首次非贵金属钴催化碳环化/芳基化反应,获得了具有高区域和非对映选择性以及广泛官能团相容性的顺式二取代吡咯。此外,还提出了亚克级实验和下游转化。
更新日期:2024-12-03
中文翻译:

钴催化的 1,5-双丙烯的区域和非对映选择性碳环化/芳基化
化丙烯的过渡金属催化碳环化是构建结构多样的环状化合物的通用方法。由于控制反应反应性和选择性的固有困难,双烯烃的碳环化研究较少。在本文中,我们报道了 1,5-双烯与芳基硼酸的首次非贵金属钴催化碳环化/芳基化反应,获得了具有高区域和非对映选择性以及广泛官能团相容性的顺式二取代吡咯。此外,还提出了亚克级实验和下游转化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号